Zine and Some Related Phenothiazines in Reducing the Rigidity of the Decerebrate Cat and in Some Other Central Actions by Elizabeth M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Schizophrenia Adverse Drug Interactions Through a Network Approach and Drug Classification Jingchun Sun University of Nashville
Virginia Commonwealth University VCU Scholars Compass Psychiatry Publications Dept. of Psychiatry 2013 Characterization of Schizophrenia Adverse Drug Interactions through a Network Approach and Drug Classification Jingchun Sun University of Nashville Min Zhao Vanderbilt University School of Medicine Ayman H. Fanous Virginia Commonwealth University Zhongming Zhao Virginia Commonwealth University Follow this and additional works at: http://scholarscompass.vcu.edu/psych_pubs Copyright © 2013 Jingchun Sun et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Downloaded from http://scholarscompass.vcu.edu/psych_pubs/10 This Article is brought to you for free and open access by the Dept. of Psychiatry at VCU Scholars Compass. It has been accepted for inclusion in Psychiatry Publications by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Hindawi Publishing Corporation BioMed Research International Volume 2013, Article ID 458989, 10 pages http://dx.doi.org/10.1155/2013/458989 Research Article Characterization of Schizophrenia Adverse Drug Interactions through a Network Approach and Drug Classification Jingchun Sun,1,2 Min Zhao,1 Ayman H. Fanous,3,4 and Zhongming Zhao1,2,5,6 1 Department of Biomedical Informatics, Vanderbilt University School of Medicine, Nashville, TN 37203, USA 2 Center for Quantitative Sciences, Vanderbilt -

Supplementary Table S1: Search Strategy for Ovid MEDLINE Electronic Database Search
Supplementary Table S1: Search strategy for Ovid MEDLINE electronic database search Database(s): Ovid MEDLINE: Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® 1946-Present Search Strategy: # Searches 1 exp "schizophrenia spectrum and other psychotic disorders"/ (psychotic disorder* or schizo* or psychosis or psychotic* or first episode or antipsychotic naive or 2 untreated or unmedicated).tw,kf. 3 Antipsychotic Agents/ or (antipsychotic* or neuroleptic*).tw,kf. 4 Chlorpromazine.mp. or Chlorpromazine/ 5 Chlorprothixene.mp. or Chlorprothixene/ 6 Droperidol.mp. or Droperidol/ 7 Flupentixol.mp. or Flupenthixol/ 8 Fluphenazine.mp. or Fluphenazine/ 9 Haloperidol.mp. or Haloperidol/ 10 (Levomepromazine or Methotrimeprazine).mp. or Methotrimeprazine/ 11 Loxapine.mp. or Loxapine/ 12 Mesoridazine.mp. or Mesoridazine/ 13 Molindone.mp. or Molindone/ 14 Periciazine.mp. 15 Pimozide.mp. or Pimozide/ 16 Prochlorperazine.mp. or Prochlorperazine/ 17 Promazine.mp. or Promazine/ 18 Thioproperazine.mp. 19 Thioridazine.mp. or Thioridazine/ 20 Thiothixene.mp. or Thiothixene/ 21 Trifluoperazine.mp. or Trifluoperazine/ 22 (Zuclopenthixol or Clopenthixol).mp. or Clopenthixol/ 23 (antipsychotic* adj5 (typical or first generation)).tw,kf. 24 (amisulpride or solian).mp. 25 ARIPIPRAZOLE/ or (aripiprazol or aripiprazole or abilify or OPC 14597).mp. 26 (asenapine or saphris or sycrest).mp. 27 (blonanserin or lonasen).mp. 28 Brexpiprazole.mp. 29 CLOZAPINE/ or (cloazpine or clozaril or leponex).mp. 30 (iloperidone or fanapt or fanapta).mp. 31 Lurasidone Hydrochloride/ or (lurasidone or latuda or sm 13496).mp. 32 (melperone or buronil).mp. 33 (olanzapine or zyprexa).mp. 34 Paliperidone Palmitate/ or (paliperidone or invega or r 76477).mp. 35 (perospirone or lullan).mp. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Chlorpromazine(BAN, Rinn)
Carpipramine Hydrochloride/Chlorpromazine 969 2. Stubb S, et al. Fixed drug eruptions: 77 cases from 1981 to 1985. Chlorpromazine Embonate (BANM, rINNM) mazine. Photosensitivity reactions are more common Br J Dermatol 1989; 120: 583. 3. Roujeau J-C, et al. Medication use and the risk of Stevens-John- Chlorpromazine, Embonate de; Chlorpromazine Pamoate; with chlorpromazine than with other antipsychotics. son syndrome or toxic epidermal necrolysis. N Engl J Med 1995; Chlorpromazini Embonas; Embonato de clorpromazina. Haematological disorders, including haemolytic anae- 333: 1600–7. Хлорпромазина Эмбонат mia, aplastic anaemia, thrombocytopenic purpura, Porphyria. Chlormezanone has been associated with acute at- (C H ClN S) ,C H O = 1026.1. tacks of porphyria and is considered unsafe in porphyric patients. 17 19 2 2 23 16 6 eosinophilia, and a potentially fatal agranulocytosis ATC — N05AA01. have occasionally been reported; they may be manifes- Preparations ATC Vet — QN05AA01. tations of a hypersensitivity reaction. Most cases of Proprietary Preparations (details are given in Part 3) agranulocytosis have occurred within 4 to 10 weeks of Chile: Cardiosedantol; Restoril†. Chlorpromazine Hydrochloride (BANM, rINNM) starting treatment, and symptoms such as sore throat or Multi-ingredient: Chile: Adalgen†; Calmosedan; Diapam; Dioran†; Dol- Aminazine; Chloropromazyny chlorowodorek; Chlorpromazin nix; Dolonase; Dolorelax†; Fibrorelax; Mesolona†; Multisedil; Neo Butar- fever should be watched for and white cell counts insti- trol; Promidan; Sedantol; Sedilit; Silrelax†; Sin-Algin; Hong Kong: Parazone; hydrochlorid; Chlorpromazine, chlorhydrate de; Chlorpromazini S.Afr.: Myoflex. hydrochloridum; Chlorpromazino hidrochloridas; Hidrocloruro tuted should they appear. Mild leucopenia has been de clorpromazina; Klooripromatsiinihydrokloridi; Klorpromazin stated to occur in up to 30% of patients on prolonged Hidroklorür; Klórpromazin-hidroklorid; Klorpromazinhydro- high dosage. -
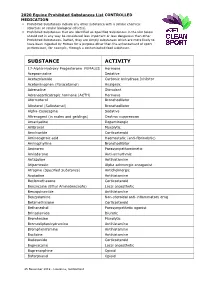
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

Gc/Ms Assays for Abused Drugs in Body Fluids
GC/MS ASSAYS FOR ABUSED DRUGS IN BODY FLUIDS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alchol, Drug Abuse, and Mental Health Administration GC/MS Assays for Abused Drugs in Body Fluids Rodger L. Foltz, Ph.D. Center for Human Toxicology University of Utah Salt Lake City, Utah 64112 Allison F. Fentiman, Jr., Ph.D. Ruth B. Foltz Battelle Columbus Laboratories Columbus, Ohio 43201 NIDA Research Monograph 32 August 1980 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse Division of Research 5600 Fishers Lane Rockville, Maryland 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 The NIDA Research Monograph series is prepared by the Division of Research of the National Institute on Drug Abuse. Its primary objective is to provide critical reviews of research problem areas and techniques, the content of state-of-the- art conferences, integrative research reviews and significant original research. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisory Board Avram Goldstein, M.D. Addiction Research Foundation Palo Alto, California Jerome Jaffe, M.D. College of Physicians and Surgeons Columbia University, New York Reese T. Jones, M.D. Langley Porter Neuropsychiatric Institute University of California San Francisco, California William McGlothlin, Ph.D. Department of Psychology, UCLA Los Angeles, California Jack Mendelson, M.D. Alchol and Drug Abuse Research Center Harvard Medical School Mclean Hospital Belmont, Massachusetts Helen Nowlis, Ph.D. Office of Drug Education, DHHS Washington, D.C. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -
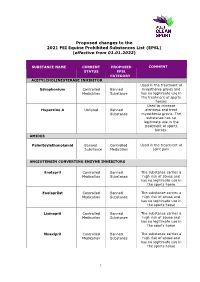
Proposed Changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (Effective from 01.01.2022)
Proposed changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (effective from 01.01.2022) SUBSTANCE NAME CURRENT PROPOSED COMMENT STATUS EPSL CATEGORY ACETYLCHOLINESTERASE INHIBITOR Used in the treatment of Edrophonium Controlled Banned myasthenia gravis and Medication Substance has no legitimate use in the treatment of sports horses Used to increase Huperzine A Unlisted Banned alertness and treat Substance myasthenia gravis. The substance has no legitimate use in the treatment of sports horses. AMIDES Palmitoylethanolamid Banned Controlled Used in the treatment of Substance Medication joint pain ANGIOTENSIN CONVERTING ENZYME INHIBITORS Enalapril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Enalaprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Lisinopril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Moexipril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse 1 Perindoprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse ANTIHISTAMINES Antazoline Controlled Banned The substance has no Medication Substance legitimate use in the sports horse Azatadine Controlled Banned The substance has Medication Substance sedative effects -

Withdrawal Versus Continuation of Long-Term Antipsychotic Drug Use for Behavioural and Psychological Symptoms in Older People with Dementia (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ghent University Academic Bibliography Cochrane Database of Systematic Reviews Withdrawal versus continuation of long-term antipsychotic drug use for behavioural and psychological symptoms in older people with dementia (Review) Van Leeuwen E, Petrovic M, van Driel ML, De Sutter AIM, Vander Stichele R, Declercq T, Christiaens T Van Leeuwen E, Petrovic M, van Driel ML, De Sutter AIM, Vander Stichele R, Declercq T, Christiaens T. Withdrawal versus continuation of long-term antipsychotic drug use for behavioural and psychological symptoms in older people with de- mentia. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No.: CD007726. DOI: 10.1002/14651858.CD007726.pub3. www.cochranelibrary.com Withdrawal versus continuation of long-term antipsychotic drug use for behavioural and psychological symptoms in older people with dementia (Review) Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 Figure1. ..................................... 8 BACKGROUND .................................... 8 OBJECTIVES ..................................... 9 METHODS ...................................... 9 RESULTS....................................... 12 Figure2. ..................................... 13 Figure3. ....................................