April 25, 1961 V M H, BOYER 2,981,616 'Li "'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NSF Renewal Proposal Appendix B
Appendix B – Part II Results from Prior Support (one-pagers) As examples of the breadth and vitality of the scientific achievements of the COMPRES community, Appendix B of this proposal provides 143 research “one-pagers” that have been submitted by research groups in preparation of this proposal that summarize selected published results enabled by COMPPRES investment in community facilities and infrastructure from 2012- 2016. B-1 Table of Contents – One-Pagers High pressure-temperature phase diagrams of energetic materials: FOX-7 & TATB B-6 Nanocrystals in compression: Unexpected structural phase transition and amorphization due to surface impurities B-7 Pressure induced conductivity and yellow-to-black piezochromism in a layered Cu-Cl hybrid perovskite B-8 High-pressure single-crystal structures of 3D lead-halide hybrid perovskites and pressure effects on their electronic and optical properties B-9 Pressure-driven high-to-low spin transition in the bimetallic quantum magnet [Ru2(O2CMe)4]3[Cr(CN)6] B-10 Pressure-induced magnetic crossover driven by hydrogen bonding in CuF2(H2O)2(3- chloropyridine) B-11 Pressure-induced structural transition in copper pyrazine dinitrate and implications for quantum magnetism B-12 High pressure vibrational properties of WS2 nanotubes B-13 Infrared study of the pressure-induced insulator-to-metal transition in PrRu4P12 B-14 Material and elastic properties of Al-tobermorite in ancient Roman seawater concrete B-15 High-pressure behavior of osmium: An analog for iron in Earth’s core B-16 Pressure-induced phase -
![United States Patent [191 [111 3,768,865 [45] Oct](https://docslib.b-cdn.net/cover/6344/united-states-patent-191-111-3-768-865-45-oct-2196344.webp)
United States Patent [191 [111 3,768,865 [45] Oct
United States Patent [191 [111 3,768,865 [45] Oct. 30, 1973 Dehn [54] PROCESS FOR THE DISPOSAL OF SALT SOLUTIONS CONTAMINATED WITH [56] References Cited AZIDE ‘ UNITED STATES PATENTS [75] Inventor: Frederick C. Dehn, New 2,348,16l 5/1944 Van DUZCC ................... .. 166/305 D Martinsville, W. Va. 3,l96,6l9 7/l965 Shock . .. 6l/.5 X 3,135,50l 6/1964 Dahms et al .......................... .._299/4 [731 Assignee: PPG Industries, Inc., Pittsburgh, Pa. [221 Filed: July 13, 1972 Primary Examiner--Ernest R. Purser Attorney-Russell A. Eberly [211 Appl. No.: 271,436 Related 0.8. Application Data [57] ABSTRACT [63] Continuation-impart of Ser. No. 92,37], Nov. 24, 1970, abandoned. The disposal of salt solutions contaminated with azide is accomplished by depositing the solution in a subter U-S- Cl ------------------------- -- 299/4, 23/157, 61/0-5, ranean cavity, maintaining the solution in the subter [52] 166/305 D ranean cavity until it is essentially free of azide, and [51] Int. Cl ........................................... .. E2lb pumping the essentially azidefree solution to the gun [53] Field of Search ..................................... .. 61/05; face - 23/157-163, 190, 191, 356, 360;166/305 299/4, 5;D 18 v Claims,_ 2 Drawing_ Figures_ 39 38 ‘a 341 1\ 4 45 Na. N3 Aun No.0“ Lew-mun] HCl 16- |'——W 4~ 4.0 _ b ,42 : " l2‘ ~ ' <—-sTeAm l4 _' _ KNS. DRIPS Patented‘ Oct. 30, 1973 3,768,865 2 Sheets-Sheet 1 V =03:nzaz8: $502563 p.01. INVENTOR FPEDfk/CK c. UEHN ATTORNEYj Patented Oct. 30, 1973 3,768,865.‘ 2 Sheets-Sheet >3 mom/m c. -

Thermophysics of Alkali and Related Azides II. Heat Capacities of Potassium, Rubidium, Cesium, and Thallium Azides from 5 to 350 K E,B
A-046 J. Chem. Thermodynamics 1978, 10, 1181-1200 Thermophysics of alkali and related azides II. Heat capacities of potassium, rubidium, cesium, and thallium azides from 5 to 350 K e,b ROBERT W. CARLING cud and EDGAR F. WESTRUM, JR.” Department of Chemistry, University of Michigan, Ann Arbor, Michigan 48109, U.S.A. (Received 26 July I977; in revised form 7 April 1978) The heat capacities of potassium, rubidium, &urn, and thallium azides were determined from 5 to 350 K by adiabatic calorimetry. Although the alkali-metal azides studied in this work exhibited no thermal anomalies over the temperature range studied, thaIlium azide has a bifurcated anomaly with two maxima at (233.0*0.1) K and (242.0410.02) K. The associated excess entropy was 0.90 calth K-l mol-I. The thermal properties of the azides and the corresponding structurally similar hydrogen difluorides are nearly identical. Both have linear symmetrical anions. However, thallium azide shows a solid-solid phase transition not exhibited by thallium hydrogen difluoride. At 298.15 K the values of C& S”, and -{G”(T)-HN”(0)}/T, respectively, are 18.38, 24.86, and 12.676 calth K-l mol-r for potassium azide; 19.09,28.78, and 15.58 calth K-l mol-1 for rubidium azide; 19.89, 32.11, and 18.17 calth K-l mol-’ for cesium azide; and 19.26, 32.09, and 18.69 calth K-l mol-’ for thallium azide. Heat capacities at constant volume for KNB were deduced from infrared and Raman data. -

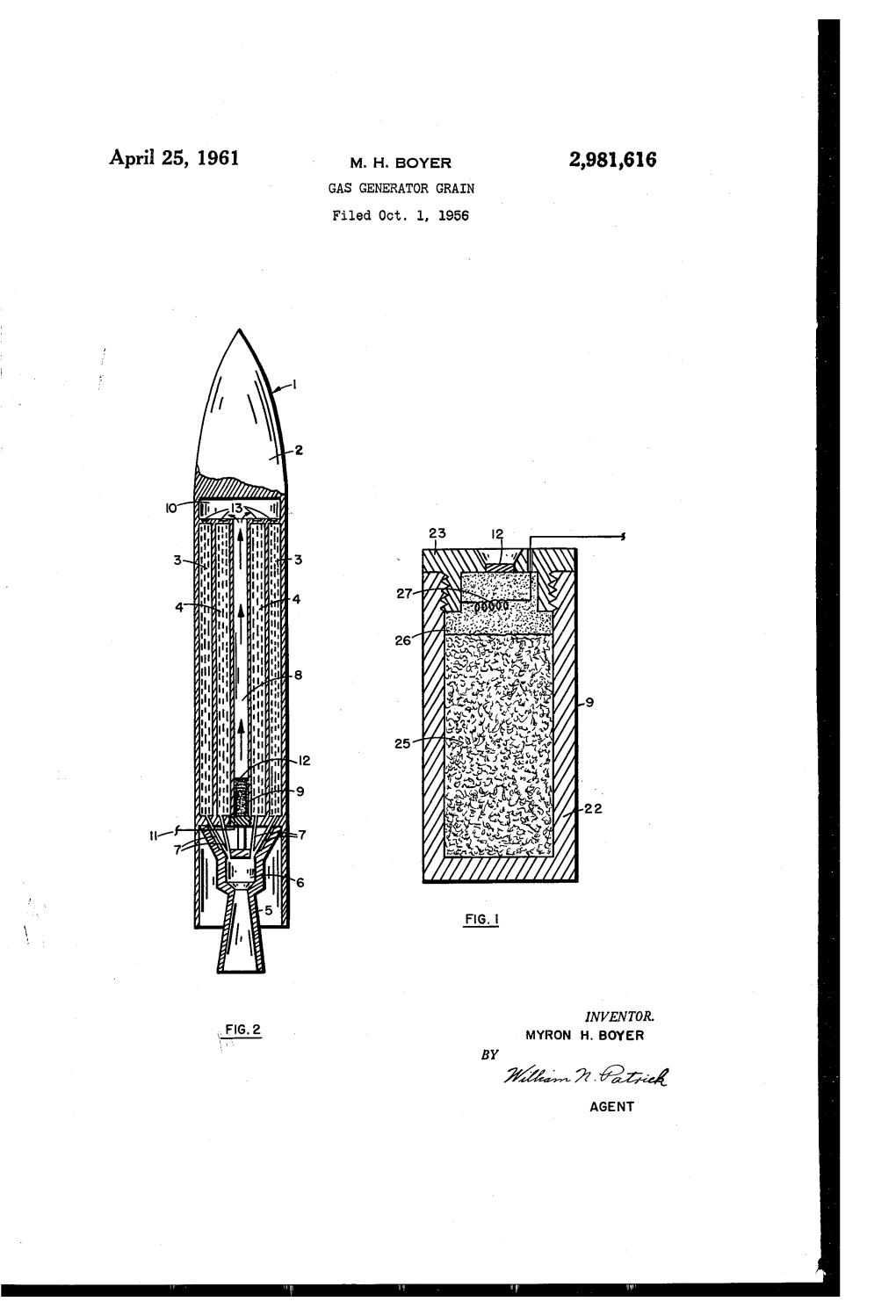
Inventor. Myron H. Boyer
INVENTOR. MYRON H. BOYER BY (2=4 AGEN United States Patent Office is and x represents the valency of M, and (2) at least one oxidizing compound selected from the class consisting of metal peroxides, inorganic perchlorates and metal ni 2,981,616 trates; said azides and said oxidizing compounds being present in amounts expressed by the equation n/my, GAS GENERATOR GRAN wherein in represents the number of azide nitrogenatoms, Myron H. Boyer, Puente, Calif., assignor to m represents the number of equivalents of oxidizing comi North American Aviation, Inc. pounds, and y has a numerical value of from about 23 to about 3.6. |- Filed Oct. 1, 1956, Ser. No. 613,327 10 An example of the gas generator grain of this inven 8 Claims. (CI.52-5) tion is a composition comprising calcium azide and pos tassium perchlorate in amounts such that the “equatiòn n/n has the value 3. The azides, M(Na), that can be used in the prepara This invention relates to a novel composition of matter 5 tion of the compositions of this invention can be any suitable for generating gases. More particularly, this azide which has suitable stability to permit grinding and invention relates to a composition which upon ignition handling in the preparation of the composition, and also generates an inert gas useful for pressurizing rocket pro will burn at a satisfactory rate without exploding. A pellant tanks. class of metal azides which satisfy these requirements In a rocket or missile which is operated on liquid fuel, 20 are the alkali and alkaline earth azides. -

CHEM Safety Manual
Department of Chemistry Safety Manual October 2018 Safety Committee Department of Chemistry Hong Kong University of Science and Technology Table of Contents 1.0 Introduction 2.0 Safety Policy and Responsibility for Safety 2.1 Department Head 2.2 Department of Chemistry Safety Committee 2.3 Laboratory Supervisors 2.4 Researchers 2.5 HSEO 3.0 Information, Training, Safety Clearance, and Safety Clearance at Termination 3.1 Initial Training 3.2 Information on Hazardous Substances 3.3 Additional Safety Information 3.4 Safety Clearance at Termination 4.0 Personal Protective Equipment and Safety Engineering Controls 4.1 Eye Protection 4.2 Protective Apparel 4.3 Respirators 4.4 Laboratory Fume Cupboards 4.5 Fire Extinguishers, Safety Showers, and Eyewash Facilities 5.0 Standard Operating Procedures for Work with Hazardous Substances 5.1 Classes of Hazardous Substances 5.2 General Procedures for Work with Toxic Substances 5.3 General Procedures for Work with Flammable and Explosive Substances 6.0 Procedures for Work with Particularly Hazardous Substances 6.1 Identification and Classification of Particularly Hazardous Substances 6.2 Designated Areas 6.3 General Procedures for Work with Substances of Moderate to High Chronic or High Acute Toxicity 6.4 Additional Procedures for Work with Substances of Known High Chronic Toxicity 6.5 Specific Handling Procedures for Some Common Particularly Hazardous Substances 7.0 Proper Planning of Laboratory Work 7.1 Recognition and Assessment 7.2 Planning for the Unexpected: What Could Go Wrong? 7.3 Site Selection -
Material Safety Data Sheet
Material Safety Data Sheet Carbon Disulfide, 99.9%, Spectrophotometric Grade ACC# 95965 Section 1 - Chemical Product and Company Identification MSDS Name: Carbon Disulfide, 99.9%, Spectrophotometric Grade Catalog Numbers: AC167710000, AC167715000 Synonyms: Carbon Bisulfide; Dithiocarbonic Anhydride; Sulphocarbonic Anhydride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 75-15-0 Carbon Disulfide >99% 200-843-6 Hazard Symbols: T F Risk Phrases: 11 36/38 48/23 62 63 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: clear, colorless liquid. Flash Point: -22 deg F. Danger! Extremely flammable liquid. May cause central nervous system effects. This substance has caused adverse reproductive and fetal effects in animals. Causes severe eye irritation. May cause skin irritation. May cause respiratory and digestive tract irritation. May cause liver and kidney damage. May cause cardiac disturbances. May cause skin sensitization by skin contact. Target Organs: Kidneys, liver, cardiovascular system, nervous system. Potential Health Effects Eye: May cause severe eye irritation. Skin: Causes skin irritation. May be absorbed through the skin in harmful amounts. Prolonged and/or repeated contact may cause defatting of the skin and dermatitis. Dermatitis and vesiculation may result from skin contact with the vapor or liquid. Chronic exposure may result in sensitization. Ingestion: May cause digestive tract disturbances. May cause effects similar to those for inhalation exposure. Can cause nervous system damage. Ingestion may cause convulsions, seizures and possible coma. -

Report-Of Committee on Chemicals and Explosives
448 REPORT OF COMMITTEE ON CHEMICALS AND EXPLOSIVES CE-1 Report-of Committee on Chemicals and Explosives Correlating Committee Dr. Robert W. Van Dolah, Chairman, Pittsburgh Mining and Safety Research Center, Bureau of Mines, U.S. Department of the Interior, 4800 Forbes Ave., Pittsburgh, PA 15213 Chester I. Babeock,~ Secretary, National Pire Protection Assn., 470 Atlantic Ave., Boston, MA 02210 W. H. Doyle, Simsbury, CT ilenry T. Rlttman, Institute of Makers of •, Thomas E. Duke, Fire Prevention & Engi- Explosives neering Bureau of Texas Richard F. Schwab, Allied Chemical Corp. Dr. Richard Y. Le Vine, Olin Corp. tNonvoting. Sectional Committee on Electrical Equipment in Chemical Atmospheres Dr. Richard Y. Le Vine, Chairman, Olin Corp., 120 Long Ridge Rd., Stamford, CT 06904 Chester I. Babcock,~ Secretary, National Fire Protection Association, 470 Atlantic Ave., Boston, MA 02210 L. J. Hall. Panel No. 14, National Electrical R. F. Schwab, Morristown, NJ Code Committee W. A. Short, National Electrical-Manu- • Robert P. llowell, American Petroleu~i In" facturers Assn. stitute George O. Hunt, Jr., Manufacturing Chem- Alternates. ists' Assn. Elton L. Lltehfleld, Pittsburgh, PA F. D. Alroth. (Alternate to P. J. Schram) Frederick L. Maltby, Instrument Society W. Calder (Alternate to F. L. Maltby) of America W. H. Levers (Alternate to Robert P. C. E. Miller, Norwood, MA Howell) " Frank E. Rademacher, Chicago, IL J. Rennle (Alternate to C. E. Miller) John E. Rogerson. Cincinnati, OH Thomas S. Staron, (Alternate to Frank E. P. J. Schram, Chicago, IL Rademaehcr) tNonvoting 449 CE-2 EXPLANATION OF REPORT Sectional Committee on llazardous Chemical Reactions R. F. Schwab, Chairman, Allied Chemical Corp., P.O. -

731 2964292 4:22: §::1;"::,:%I';;;~ 112/22 9 Montreal’ Quebm Canada 3,797,854 3/1974 Poole Et Al
United States Patent [19] 1111 3,883,373 Sidebottom [45] May 13, 1975 [54] GAS GENERATING COMPOSITIONS [56] References Cited [75] Inventor: Eric William Sidebottom, Otterburn UNITED STATES PATENTS Heights, Quebec. Canada 2,981,616 4/1961 Boyer .................................. .. 149/35 [731 2964292 4:22: §::1;"::,:%i';;;~ 112/22 9 Montreal’ Quebm Canada 3,797,854 3/1974 POOle et al ...................... .. 149/35 x [22] Filed: July 2, 1973 _ Primary Examiner—Stephen J. Lechert, Jr. [2]] Appl' NO" 375’654 Attorney, Agent, or Firm—Alexander O. Mclntosh [30] Foreign Application Priority Data [57] ABSTRACT July 24, i972 United Kingdom ............. .. 34481/72 A gas generating composition having as ingredients an . _ alkali or alkaline earth metal azide, an oxidizing com [52] US. Cl. ...................... .. 149/6; 149/35, 149/37, pound, an Oxide such as silica or alumina, and option_ 149/40; 149/41; 149/42; 149/43;_ 149/45; ally, a metal such as silicon or aluminum. The compo 149/75’ 149/77 sition is useful as a source of gas to in?ate bags used Int. _ . ..' . I . _ . .. as restraint Systems for the protection of automobile [58] Field of Search ............. .. 149/6, 35, 37, 75, 40, passengers. 149/77, 45, 36, 42, 41, 43 8 Claims, No Drawings 3,883,373 1 2 GAS GENERATING COMPOSITIONS and zinc, in proportion sufficient to react with the me tallic residue of the reaction between (1) and (2). This invention relates to a composition of matter suit The use of a mixture of an oxide and a metal as ingre able for generating gases. -

CSEM Scientific and Technical Report 2016
SCIENTIFIC AND TECHNICAL REPORT 2016 CSEM SA Jaquet-Droz 1 CH-2002 Neuchâtel CSEM Alpnach Untere Gründlistrasse 1 CH-6055 Alpnach Dorf CSEM Landquart Bahnhofstrasse 1 CH-7302 Landquart CSEM Muttenz Tramstrasse 99 CH-4132 Muttenz CSEM Zurich Technoparkstrasse 1 CH-8005 Zurich www.csem.ch [email protected] [email protected] IMPRINT Full report title CSEM Scientific and Technical Report 2016 Editor and publisher CSEM SA [email protected] T +41 32 720 5111 Design CSEM Communications [email protected] T +41 32 720 5111 Publication Frequency yearly Media printed and electronic Website www.csem.ch/str2016 Printing Imprimerie Baillod SA, Bevaix (Switzerland) Cover page Projection of artwork by coherent illumination of designed optical nanostructures. The project creates a connection between nanotechnology and art by using designed nanostructures to project artwork in single and full color. Once the optical nanostructures have been designed and a physical master created, an infinite number of low-cost copies can be reproduced. The original artwork, from the artist Véronique Lecomte, known as Velec, is made of neon light tubes. Courtesy: VELEC www.velec.ch CSEM SA CSEM is a private, non-profit research and technology organization (RTO) and a Swiss innovation accelerator—a catalyst for the transfer of technologies and know-how from fundamental research to industry. CSEM’s extraordinary success in the field of innovation and its diffusion stems from its promotion of technology-based activities that address the next generation of trends—the future of energy, the Internet of Things revolution, the advent of wearable technologies for wellness and medical applications, or the rendering widespread of machine-to- machine (M2M) communications required by Industry 4.0. -

Formation Mechanism of Insensitive Tellurium Hexanitride with Armchair
ARTICLE https://doi.org/10.1038/s42004-020-0286-1 OPEN Formation mechanism of insensitive tellurium hexanitride with armchair-like cyclo-N6 anions ✉ ✉ Zhao Liu1,DaLi 1 , Quan Zhuang1, Fubo Tian1, Defang Duan 1, Fangfei Li1 & Tian Cui1,2 1234567890():,; The lower decomposition barriers of cyclo-N6 anions hinder their application as high-energy- density materials. Here, first-principles calculations and molecular dynamics simulations reveal that enhancing the covalent component of the interaction between cyclo-N6 anions and cations can effectively improve the stability of cyclo-N6 anions. Taking tellurium hex- anitride as a representative, the exotic armchair-like N6 anions of tellurium hexanitride exhibit resistance towards electronic attack and gain extra stability through the formation of covalent bonds with the surrounding elemental tellurium under high pressures. These covalent bonds effectively improve the chemical barrier and insensitivity of tellurium hexanitride during blasting, which prevents the decomposition of solid cyclo-N6 salts into molecular nitrogen. Furthermore, the high-pressure induced covalent bonds between cyclo-N6 anions and tell- urium enable the high bulk modulus, remarkable detonation performance, and high- temperature thermodynamic stability of tellurium hexanitride. 1 State Key Laboratory of Superhard Materials, Jilin University, Changchun 130012, People’s Republic of China. 2 School of Physical Science and Technology, ✉ Ningbo University, Ningbo 315211, People’s Republic of China. email: [email protected]; [email protected] COMMUNICATIONS CHEMISTRY | (2020) 3:42 | https://doi.org/10.1038/s42004-020-0286-1 | www.nature.com/commschem 1 ARTICLE COMMUNICATIONS CHEMISTRY | https://doi.org/10.1038/s42004-020-0286-1 igh pressure, a typically clean and controllable thermo- with nitrogen; thus, we adopt the binary Te–N candidates as dynamic variable, can be adopted to obtain curious prototypes to search for cyclo-N6 ions and study the trap effect by H fi 27,28 materials that are dif cult to synthesize under ambient the covalent bond . -

Energetic Materials 1
ENERGETIC MATERIALS Energetic Materials 1 Volume 1 : Physics and Chemistry of the Inorganic Azides Volume 2: Technology of the Inorganic Azides Physics and Chemistry of the Inorganic Azides Edited by H. [I. Fair :1nC1 R. F. W;~lkcr Energetic Materiuh Division Armanrent Research and Development Command Dover, New Jersey PLENUM PRESS NEW YORK AND LONDON and b + i. Some of the reactions are evidently complex, and such equations &$@[ The same author 1241 also obtained HN3 from hydrazine and hydroxylamine by oxidation (b + c): Lm text are not strictly quantitative. i 1 laterCu , discovered HN3 [I] by reacting benzoylhydrazine with nitrous acid i N2H4 + NHzOH + 20 4 HN3 + 3H2O (b + g-type reaction). The resulting benzoyl azide was saponified, and sodium azide was isolated from the alkaline mixture. In 1903 Wislicenus [14] synthesized Of various oxidants tested, hydroperoxide and chromic acid gave the best sodium azide solely from inorganic compounds, namely, sodium metal, am- results (24% yield). monia, and dinitrogen oxide (a + e). The reaction proceeds in two steps, first Thiele [25] converted aminoguanidine with nitrous acid (b + g) to guanyl converting ammonia with sodium to sodamide, and then reacting this with di- azide [26] which was saponified to sodium azide: nitrogen oxide to yield sodium azide. Fifty percent of the sodamide is decom- posed to hydroxide and ammonia, and the overall balance of the process is 2NaNH2 + N20 -+ NaN3 + NaOH + NH3 Wislicenus conducted the synthesis as a dry procedure at elevated temperature; a Another interesting reaction (b + g) was studied by Freund and Schander low-temperature procedure in liquid ammonia was later patented by Acken and [?7]. -

III.I.III III US005523424A United States Patent (19) 11 Patent Number: 5,523,424 Malik Et Al
III.I.III III US005523424A United States Patent (19) 11 Patent Number: 5,523,424 Malik et al. (45) Date of Patent: Jun. 4, 1996 (54) SOLVENT-FREE PROCESS FOR THE 4,707,540 11/1987 Manser et al. .......................... 528/417 SYNTHESIS OF ENERGETIC OXETANE 4,764,586 8/1988 Manser et al. ... 149/19.6 MONOMERS 4,804,424 2/1989 Hinshaw ............ ... 149/19.6 4,938,814 7/1990 Schonyer et al... ... 149/19.9 (75) Inventors: Aslam A. Malik, Cameron Park; 4,952,952,644 8/1990 Wardle et al. .......................... 525/410 Gerald E. Manser, El Dorado Hills; 5,049,214 9/1991 Hassell et al. ....................... 149/09.4 Roland P. Carson; Thomas G. FOREIGN PATENT DOCUMENTS Archibald, both of Fair Oaks, all of Calif. 223679 1/1968 Russian Federation. 1112496 5/1968 United Kingdom. (73) Assignee: Aerojet-General Corporation, Rancho Primary Examiner-C. Warren Ivy Cordova, Calif. Assistant Examiner-Amelia Owens Attorney, Agent, or Firm-Townsend and Townsend and 21 Appl. No.: 334,708 Crew (22) Filed: Nov. 4, 1994 57 ABSTRACT 51 Int. C. a - C07D 305/00 This invention relates to a process for the preparation of 52 U.S. Cl. ..................................... 549/510 mono- and bis(azidomethyl)oxetanes. These compounds are 58 Field of Search ............................................... 549/510 prepared by combining a mono- or bis-X-oxetane (wherein Xis, for example, a tosylate, mesylate, halogen, etc.) with an (56) References Cited aqueous solution of a metallic azide and a phase transfer U.S. PATENT DOCUMENTS catalyst. Mono- and bis(azidiomethyl)oxetanes can be poly merized to form homopolymers and copolymers with load 3,138,609 11/1964 Carpenter ...............................