Explosive Basaltic Volcanism of the Chikurachki Volcano (Kurile Arc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2002 Volcanic Activity in Alaska and Kamchatka: Summary of Events and Response of the Alaska Volcano Observatory by Christina A
2002 Volcanic Activity in Alaska and Kamchatka: Summary of Events and Response of the Alaska Volcano Observatory by Christina A. Neal, Robert G. McGimsey, and Olga Girina Open-File Report 2004-1058 U.S. Department of the Interior U.S. Geological Survey 2002 Volcanic Activity in Alaska and Kamchatka: Summary of Events and Response of the Alaska Volcano Observatory By Christina A. Neal1, Robert G. McGimsey1, and Olga Girina2 1Alaska Volcano Observatory, 4200 University Dr., Anchorage, AK 99508-4664 2Kamchatka Volcanic Eruptions Response Team, Institute of Volcanic Geology and Geochemistry, Piip Blvd, 9 Petropavlovsk- Kamchatsky, 683006, Russia AVO is a cooperative program of the U.S. Geological Survey, University of Alaska Fairbanks Geophysical Institute, and the Alaska Division of Geological and Geophysical Surveys. AVO is funded by the U.S. Geological Survey Volcano Hazards Program and the State of Alaska Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government Open-File Report 2004-1058 U.S. Department of the Interior U.S. Geological Survey TABLE OF CONTENTS Introduction..................................................................................................................................1 Volcanic.Activity.in.Alaska..........................................................................................................4 Wrangell.Volcano.........................................................................................................................4 -

The Active Volcanoes of Kamchatka and Paramushir Island, North Kurils in 2007 O
ISSN 0742-0463, Journal of Volcanology and Seismology, 2009, Vol. 3, No. 1, pp. 1–17. © Pleiades Publishing, Ltd., 2009. Original Russian Text © O.A. Girina, S.V. Ushakov, N.A. Malik, A.G. Manevich, D.V. Mel’nikov, A.A. Nuzhdaev, Yu.V. Demyanchuk, L.V. Kotenko, 2009, published in Vul- kanologiya i Seismologiya, 2009, No. 1, pp. 3–20. The Active Volcanoes of Kamchatka and Paramushir Island, North Kurils in 2007 O. A. Girina, S. V. Ushakov, N. A. Malik, A. G. Manevich, D. V. Mel’nikov, A. A. Nuzhdaev, Yu. V. Demyanchuk, and L. V. Kotenko Institute of Volcanology and Seismology of the Far East Division of the Russian Academy of Sciences, 9 Piipa Boulevard, Petropavlovsk-Kamchatskii, 683006 Russia Received March 6, 2008 Abstract—Eight strong eruptions of four Kamchatka volcanoes (Bezymyannyi, Klyuchevskoi, Shiveluch, and Karymskii) and Chikurachki Volcano on Paramushir Island, North Kurils took place in 2007. In addition, an explosive event occurred on Mutnovskii Volcano and increased fumarole activity was recorded on Avacha and Gorelyi volcanoes in Kamchatka and Ebeko Volcano on Paramushir Island, North Kurils. Thanks to close coop- eration with colleagues involved in the Kamchatkan Volcanic Eruption Response Team (KVERT) project from the Elizovo Airport Meteorological Center and volcanic ash advisory centers in Tokyo, Anchorage, and Wash- ington (Tokyo VAAC, Anchorage VAAC, and Washington VAAC), all necessary precautions were taken for flight safety near Kamchatka. DOI: 10.1134/S0742046309010011 INTRODUCTION two months in 2005–2006 the volcano remained rela- tively quiet, up to December 4, 2006. The extrusive– The comprehensive monitoring of active volcanoes explosive eruption of Molodoi (Young) Shiveluch Vol- in Kamchatka and North Kurils is carried out under the cano commenced on that date, with a strong ash ejec- KVERT project [2, 4, 5] in close cooperation with col- tion, and continued during all of 2007. -

USGS Open-File Report 2009-1133, V. 1.2, Table 3
Table 3. (following pages). Spreadsheet of volcanoes of the world with eruption type assignments for each volcano. [Columns are as follows: A, Catalog of Active Volcanoes of the World (CAVW) volcano identification number; E, volcano name; F, country in which the volcano resides; H, volcano latitude; I, position north or south of the equator (N, north, S, south); K, volcano longitude; L, position east or west of the Greenwich Meridian (E, east, W, west); M, volcano elevation in meters above mean sea level; N, volcano type as defined in the Smithsonian database (Siebert and Simkin, 2002-9); P, eruption type for eruption source parameter assignment, as described in this document. An Excel spreadsheet of this table accompanies this document.] Volcanoes of the World with ESP, v 1.2.xls AE FHIKLMNP 1 NUMBER NAME LOCATION LATITUDE NS LONGITUDE EW ELEV TYPE ERUPTION TYPE 2 0100-01- West Eifel Volc Field Germany 50.17 N 6.85 E 600 Maars S0 3 0100-02- Chaîne des Puys France 45.775 N 2.97 E 1464 Cinder cones M0 4 0100-03- Olot Volc Field Spain 42.17 N 2.53 E 893 Pyroclastic cones M0 5 0100-04- Calatrava Volc Field Spain 38.87 N 4.02 W 1117 Pyroclastic cones M0 6 0101-001 Larderello Italy 43.25 N 10.87 E 500 Explosion craters S0 7 0101-003 Vulsini Italy 42.60 N 11.93 E 800 Caldera S0 8 0101-004 Alban Hills Italy 41.73 N 12.70 E 949 Caldera S0 9 0101-01= Campi Flegrei Italy 40.827 N 14.139 E 458 Caldera S0 10 0101-02= Vesuvius Italy 40.821 N 14.426 E 1281 Somma volcano S2 11 0101-03= Ischia Italy 40.73 N 13.897 E 789 Complex volcano S0 12 0101-041 -

Alaska Interagency Operating Plan for Volcanic Ash Episodes
Alaska Interagency Operating Plan for Volcanic Ash Episodes August 1, 2011 COVER PHOTO: Ash, gas, and water vapor cloud from Redoubt volcano as seen from Cannery Road in Kenai, Alaska on March 31, 2009. Photograph by Neil Sutton, used with permission. Alaska Interagency Operating Plan for Volcanic Ash Episodes August 1, 2011 Table of Contents 1.0 Introduction ............................................................................................................... 3 1.1 Integrated Response to Volcanic Ash ....................................................................... 3 1.2 Data Collection and Processing ................................................................................ 4 1.3 Information Management and Coordination .............................................................. 4 1.4 Warning Dissemination ............................................................................................. 5 2.0 Responsibilities of the Participating Agencies ........................................................... 5 2.1 DIVISION OF HOMELAND SECURITY AND EMERGENCY MANAGEMENT (DHS&EM) ......................................................................................................... 5 2.2 ALASKA VOLCANO OBSERVATORY (AVO) ........................................................... 6 2.2.1 Organization ...................................................................................................... 7 2.2.2 General Operational Procedures ...................................................................... 8 -

Russia Background
The World Factbook Central Asia :: Russia Introduction :: Russia Background: Founded in the 12th century, the Principality of Muscovy, was able to emerge from over 200 years of Mongol domination (13th-15th centuries) and to gradually conquer and absorb surrounding principalities. In the early 17th century, a new Romanov Dynasty continued this policy of expansion across Siberia to the Pacific. Under PETER I (ruled 1682-1725), hegemony was extended to the Baltic Sea and the country was renamed the Russian Empire. During the 19th century, more territorial acquisitions were made in Europe and Asia. Defeat in the Russo-Japanese War of 1904-05 contributed to the Revolution of 1905, which resulted in the formation of a parliament and other reforms. Repeated devastating defeats of the Russian army in World War I led to widespread rioting in the major cities of the Russian Empire and to the overthrow in 1917 of the imperial household. The communists under Vladimir LENIN seized power soon after and formed the USSR. The brutal rule of Iosif STALIN (1928-53) strengthened communist rule and Russian dominance of the Soviet Union at a cost of tens of millions of lives. The Soviet economy and society stagnated in the following decades until General Secretary Mikhail GORBACHEV (1985-91) introduced glasnost (openness) and perestroika (restructuring) in an attempt to modernize communism, but his initiatives inadvertently released forces that by December 1991 splintered the USSR into Russia and 14 other independent republics. Since then, Russia has shifted its post-Soviet democratic ambitions in favor of a centralized semi-authoritarian state in which the leadership seeks to legitimize its rule through managed national elections, populist appeals by President PUTIN, and continued economic growth. -

Emulating Volcanic Ash Fall for Multi‑Scale Analysis:Development of The
MANILA Record 2014/36 | GeoCat 81611 Emulating volcanic ash fall for multi-scale analysis: Development of the VAPAHR tool and application to the Asia-Pacific region for the United Nations Global Assessment Report 2015 Bear-Crozier, A. N.1, Miller, V.1, Newey, V.1, Horspool, N.1 and Weber, R.1 APPLYING GEOSCIENCE TO AUSTRALIA’S MOST IMPORTANT CHALLENGES www.ga.gov.au Emulating volcanic ash fall for multi-scale analysis: Development of the VAPAHR tool and application to the Asia- Pacific region for the United Nations Global Assessment Report 2015 GEOSCIENCE AUSTRALIA RECORD 2014/36 Bear-Crozier, A. N.1, Miller, V.1, Newey, V.1, Horspool, N.1 and Weber, R.1 1. Geoscience Australia Department of Industry Minister for Industry: The Hon Ian Macfarlane MP Parliamentary Secretary: The Hon Bob Baldwin MP Secretary: Ms Glenys Beauchamp PSM Geoscience Australia Chief Executive Officer: Dr Chris Pigram This paper is published with the permission of the CEO, Geoscience Australia © Commonwealth of Australia (Geoscience Australia) 2014 With the exception of the Commonwealth Coat of Arms and where otherwise noted, all material in this publication is provided under a Creative Commons Attribution 3.0 Australia Licence. (http://www.creativecommons.org/licenses/by/3.0/au/deed.en) Geoscience Australia has tried to make the information in this product as accurate as possible. However, it does not guarantee that the information is totally accurate or complete. Therefore, you should not solely rely on this information when making a commercial decision. Geoscience Australia is committed to providing web accessible content wherever possible. -
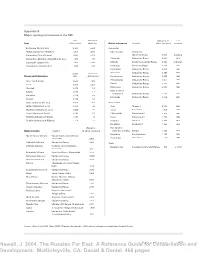
Newell, J. 2004. the Russian Far East: a Reference Guide for Conservation and Development
Appendix A Major topological features in the RFE Size Maximum Elevation (m Last Seas (000 sq. km) depth (m) Active volcanoes Location above sea level) eruption Beringovo (Bering Sea) 2,315 5,500 Kamchatka Okhotskoe (Sea of Okhotsk) 1,603 3,521 Klyuchevskoi Vostochny Yaponskoe (Sea of Japan) 1,602 3,720 (Eastern) Range 4,800 ongoing Vostochno-Sibirskoe (East Siberian Sea) 913 915 Tolbachik Vostochny Range 3,682 1976 Laptevykh (Laptev Sea) 662 3,385 Ichinsky Sredinny (Central) Range 3,621 unknown Chukotskoe (Chukchi Sea) 595 1,256 Kronotsky Gamchen Ridge 3,528 1923 Koryaksky Vostochny Range 3,456 1957 Length Basin size Shiveluch Vostochny Range 3,283 2001 Rivers and tributaries (km) (000 sq. km) Bezymyanny Vostochny Range 3,085 1998 Zhupanovsky Vostochny Range 2,927 1956 Amur (with Argun) 4,444 1,855 Avacha Vostochny Range 2,750 1991 Lena 4,400 2,490 Mutnovsky Vostochny Range 2,323 1999 Olenyok 2,270 219 Maly (Lesser) Kolyma 1,870 422 Semlyachik Vostochny Range 1,560 1952 Indigirka 1,726 360 Karymsky Vostochny Range 1,486 2001 Anadyr 1,150 191 Vilyui (tributary of Lena) 2,650 454 Kuril Islands Aldan (tributary of Lena) 2,273 729 Alaid Atlasov I. 2,339 2000 Olyokma (tributary of Lena) 1,436 210 Tyatya Kunashir I. 1,819 1973 Zeya (tributary of Amur) 1,242 233 Chikurachki Paramushir I. 1,816 1995 Markha (tributary of Vilyui) 1,181 99 Fussa Paramushir I. 1,772 1984 Omolon (tributary of Kolyma) 1,114 113 Sarychev Matua I. 1,446 1976 Krenitsyn Onekotan I. -

Alaska Interagency Operating Plan for Volcanic Ash Episodes
Alaska Interagency Operating Plan for Volcanic Ash Episodes April 1, 2004 Alaska Interagency Operating Plan for Volcanic Ash Episodes April 1, 2004 Table of Contents 1.0 INTRODUCTION ..........................................................1 1.1 INTEGRATED RESPONSE TO VOLCANIC ASH ..............................1 1.2 DATA COLLECTION AND PROCESSING ..................................2 1.3 INFORMATION MANAGEMENT AND COORDINATION ........................2 1.4 DISTRIBUTION AND DISSEMINATION ...................................3 2.0 RESPONSIBILITIES OF THE PARTIES ...........................................3 2.1 DIVISION OF HOMELAND SECURITY AND EMERGENCY MANAGEMENT (DHS&EM) . 3 2.2 ALASKA VOLCANO OBSERVATORY (AVO) .................................4 2.2.1 ORGANIZATION .................................................4 2.2.2 GENERAL OPERATIONAL PROCEDURES ..............................5 2.2.2.1 EARLY ERUPTION PREDICTION, WARNING & CALL-DOWN: SEISMICALLY INSTRUMENTED VOLCANOES ......................................6 2.2.2.1.1 KAMCHATKAN VOLCANIC ERUPTION RESPONSE TEAM (KVERT) . 8 2.2.2.2 SEISMICALLY UNMONITORED VOLCANOES ...........................9 2.2.2.3 UPDATES AND INFORMATION RELEASES .............................9 2.2.2.4 COMMUNICATION WITH OTHER AGENCIES ...........................10 2.2.2.5 LEVEL OF CONCERN COLOR CODE SYSTEM ..........................10 2.2.2.6 DESIGNATION OF AUTHORITY ....................................12 2.3 DEPARTMENT OF DEFENSE .............................................12 2.3.1 PROCEDURES .................................................12 -

Geochemical Studies of Volcanic Rocks from the Northern Part of Kuril-Kamchatka
Geochemical studies of volcanic rocks from the northern part of Kuril-Kamchatka arc: Tectonic and structural Title constraints on the origin and evolution of arc magma Author(s) Bergal-Kuvikas, Olga Citation 北海道大学. 博士(理学) 甲第11991号 Issue Date 2015-09-25 DOI 10.14943/doctoral.k11991 Doc URL http://hdl.handle.net/2115/60073 Type theses (doctoral) File Information Bergal-Kuvikas_Olga.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Thesis for Doctor of Philosophy in Petrology and Volcanology research group, Division of Earth and Planetary Science, Department of Natural History Sciences, Graduate School of Science, Hokkaido University Geochemical studies of volcanic rocks from the northern part of Kuril-Kamchatka arc: Tectonic and structural constraints on the origin and evolution of arc magma (クリル・カムチャッカ弧北部の火山岩に関する地球化学的研究: 深部構造が島弧マグマの起源および進化に与える影響について) Bergal-Kuvikas Olga Hokkaido University August 2015 Acknowledgement I am wishing to thank Japanese government for the chance to study at Japan. Scholarship for research students (Monbukagakusho: MEXT) was spent at Hokkaido University under guided by Prof. Mitsuhiro Nakagawa since October 2010 to September 2015. Especially thanks for all members of Petrology and Volcanology research group at Hokkaido University. I am grateful to Dr. Mizuho Amma-Miyasaka, Dr. Akiko Matsumoto, Ms. Ayumi Kosugi, Prof. Takeshi Kuritani for assistance and support in analytical experiences. Thanks for Mr. K. Nakamura for helpful recommendation in making thin sections. My dissertation is a part of Japanese-Russian project of study Klyuchevskoy volcano. I grateful to geologists and volcanologists of this project. For many years cooperation, I gratitude to Dr. Ya. Muravev, Ms. N. Malik, Mr. -

Geothermal Potential of the Cascade and Aleutian Arcs, with Ranking of Individual Volcanic Centers for Their Potential to Host Electricity-Grade Reservoirs
DE-EE0006725 ATLAS Geosciences Inc FY2016, Final Report, Phase I Final Research Performance Report Federal Agency and Organization: DOE EERE – Geothermal Technologies Program Recipient Organization: ATLAS Geosciences Inc DUNS Number: 078451191 Recipient Address: 3372 Skyline View Dr Reno, NV 89509 Award Number: DE-EE0006725 Project Title: Geothermal Potential of the Cascade and Aleutian Arcs, with Ranking of Individual Volcanic Centers for their Potential to Host Electricity-Grade Reservoirs Project Period: 10/1/14 – 10/31/15 Principal Investigator: Lisa Shevenell President [email protected] 775-240-7323 Report Submitted by: Lisa Shevenell Date of Report Submission: October 16, 2015 Reporting Period: September 1, 2014 through October 15, 2015 Report Frequency: Final Report Project Partners: Cumming Geoscience (William Cumming) – cost share partner GEODE (Glenn Melosh) – cost share partner University of Nevada, Reno (Nick Hinz) – cost share partner Western Washington University (Pete Stelling) – cost share partner DOE Project Team: DOE Contracting Officer – Laura Merrick DOE Project Officer – Eric Hass Project Monitor – Laura Garchar Signature_______________________________ Date____10/16/15_______________ *The Prime Recipient certifies that the information provided in this report is accurate and complete as of the date shown. Any errors or omissions discovered/identified at a later date will be duly reported to the funding agency. Page 1 of 152 DE-EE0006725 ATLAS Geosciences Inc FY2016, Final Report, Phase I Geothermal Potential of -

2008 Volcanic Activity in Alaska, Kamchatka, and the Kurile Islands: Summary of Events and Response of the Alaska Volcano Observatory
The Alaska Volcano Observatory is a cooperative program of the U.S. Geological Survey, University of Alaska Fairbanks Geophysical Institute, and the Alaska Division of Geological and Geophysical Surveys. The Alaska Volcano Observatory is funded by the U.S. Geological Survey Volcano Hazards Program and the State of Alaska. 2008 Volcanic Activity in Alaska, Kamchatka, and the Kurile Islands: Summary of Events and Response of the Alaska Volcano Observatory Scientific Investigations Report 2010–5243 U.S. Department of the Interior U.S. Geological Survey Cover: Photograph showing Okmok eruption plume as seen from Fort Glenn (ranch building in foreground) on August 3, 2008. Ash from this phase of the eruption ultimately reached the community of Nikolski on the west end of Umnak Island. Photograph by J. Larsen, AVO/UAFGI. 2008 Volcanic Activity in Alaska, Kamchatka, and the Kurile Islands: Summary of Events and Response of the Alaska Volcano Observatory By Christina A. Neal, Robert G. McGimsey, James P. Dixon, U.S. Geological Survey, Cheryl E. Cameron, Alaska Department of Geological and Geophysical Surveys, Anton A. Nuzhdaev, Institute of Volcanology and Seismology, and Marina Chibisova, Institute of Marine Geology and Geophysics The Alaska Volcano Observatory is a cooperative program of the U.S. Geological Survey, University of Alaska Fairbanks Geophysical Institute, and the Alaska Division of Geological and Geophysical Surveys. The Alaska Volcano Observatory is funded by the U.S. Geological Survey Volcano Hazards Program and the State of Alaska. Scientific Investigations Report 2010–5243 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. -

Russia´S EMERCOM
Russia has regularly experienced major natural disasters and they credibility and political emergencies Managing EMERCOM: Russias’s are becoming more frequent. This is one reason why it is of inter- est to better understand how Russia’s system for disaster man- agement and the EMERCOM are organised, have developed and manages severe emergency situations. In a relatively short time, Sergei Shoigu transformed a volunteer search and rescue team into a ministry. The EMERCOM is an impressive organisation with vast resources in personnel (including with military personnel), equipment and means of land, air and water transport. A general overview is given of natural disasters in Russia followed by a more detailed description of the widespread forest fires in 2010 and the floods in 2012–2014. This is done to analyse how EMERCOM and the Government managed these and how efficient the Russian governance system is in an emergency situation. Major disasters will challenge the political leadership and its cred- ibility if disasters are not prevented or the response is not han- dled in an efficient manner. Roger Roffey The report establishes that there are clear weaknesses in the Russia’s EMERCOM: Russian governance system for managing serious emergencies. Officials at subordinate levels are reluctant to make independent decisions for fear of being blamed by the federal authorities and Managing emergencies and will sometimes wait until they are ordered to act by higher central levels. In addition officials do not always report how serious the political credibility situation really is so as not to be blamed. This is of course very negative for handling an emergency when rapid decisions are needed to allocate resources due to how the crisis is developing in the affected area.