Neurotoxic Effects of Postnatal Thimerosal Are Mouse Strain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Program Evaluation Key Outcomes and Addressing Remaining
Vaccine 31S (2013) J73–J78 Contents lists available at ScienceDirect Vaccine jou rnal homepage: www.elsevier.com/locate/vaccine Key outcomes and addressing remaining challenges—Perspectives from a final ଝ evaluation of the China GAVI project a,1 a,1 a,1 a,1 b c Weizhong Yang , Xiaofeng Liang , Fuqiang Cui , Li Li , Stephen C. Hadler , Yvan J. Hutin , d a,∗ Mark Kane , Yu Wang a Chinese Center for Disease Control and Prevention, Beijing, China b Centers for Disease Control and Prevention, Atlanta, USA c Europe Center for Disease Control and Prevention, Stockholm, Sweden d Mercer Island, Washington, USA a r t a b i c s t l r e i n f o a c t Article history: During the China GAVI project, implemented between 2002 and 2010, more than 25 million children Received 6 June 2012 received hepatitis B vaccine with the support of project, and the vaccine proved to be safe and effective. Received in revised form 24 July 2012 With careful consideration for project savings, China and GAVI continually adjusted the budget, addi- Accepted 24 September 2012 tionally allowing the project to spend operational funds to support demonstration projects to improve timely birth dose (TBD), conduct training of EPI staff, and to monitor the project impact. Results from the final evaluation indicated the achievement of key outcomes. As a result of government co-investment, Keywords: human resources at county level engaged in hepatitis B vaccination increased from 29 per county on GAVI Project average in 2002 to 66 in 2009. All project counties funded by the GAVI project use auto-disable syringes Outcomes for hepatitis B vaccination and other vaccines. -

Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children
ORIGINAL CONTRIBUTION Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children ˚ ´ Pal Suren, MD, MPH Importance Prenatal folic acid supplements reduce the risk of neural tube defects Christine Roth, MSc in children, but it has not been determined whether they protect against other neu- Michaeline Bresnahan, PhD rodevelopmental disorders. Margaretha Haugen, PhD Objective To examine the association between maternal use of prenatal folic acid supplements and subsequent risk of autism spectrum disorders (ASDs) (autistic disor- Mady Hornig, MD der, Asperger syndrome, pervasive developmental disorder–not otherwise specified Deborah Hirtz, MD [PDD-NOS]) in children. Kari Kveim Lie, MD Design, Setting, and Patients The study sample of 85 176 children was derived from the population-based, prospective Norwegian Mother and Child Cohort Study W. Ian Lipkin, MD (MoBa). The children were born in 2002-2008; by the end of follow-up on March 31, Per Magnus, MD, PhD 2012, the age range was 3.3 through 10.2 years (mean, 6.4 years). The exposure of Ted Reichborn-Kjennerud, MD, PhD primary interest was use of folic acid from 4 weeks before to 8 weeks after the start of pregnancy, defined as the first day of the last menstrual period before conception. Synnve Schjølberg, MSc Relative risks of ASDs were estimated by odds ratios (ORs) with 95% CIs in a logistic George Davey Smith, MD, DSc regression analysis. Analyses were adjusted for maternal education level, year of birth, and parity. Anne-Siri Øyen, PhD Main Outcome Measure Specialist-confirmed diagnosis of ASDs. Ezra Susser, MD, DrPH Results At the end of follow-up, 270 children in the study sample had been diag- Camilla Stoltenberg, MD, PhD nosed with ASDs: 114 with autistic disorder, 56 with Asperger syndrome, and 100 with PDD-NOS. -

Lower Autism Risk with Folic Acid Supplements in Pregnancy 12 February 2013
Lower autism risk with folic acid supplements in pregnancy 12 February 2013 Women who took folic acid supplements in early of folic acid supplements in the mother during pregnancy almost halved the risk of having a child pregnancy and a reduced risk of childhood autism. with autism. Beginning to take folic acid supplements later in pregnancy did not reduce the "The study does not prove that folic acid risk. This is shown in new findings from the ABC supplements can prevent childhood autism. Study and Norwegian Mother and Child Cohort However, the findings are so apparent that they Study published in the Journal of The American constitute a good argument to further examine Medical Association (JAMA). possible causal mechanisms. It should also be ascertained whether folic acid is associated with a Women who took folic acid supplements from four reduced risk of other brain disorders in children," weeks before conception to eight weeks into says Surén. pregnancy had a 40 per cent lower risk of giving birth to children with childhood autism (classic Emphasises the importance of folic acid autism). Use of folic acid supplements midway supplements through pregnancy (week 22) had no effect. The results support the Norwegian Directorate of The findings only apply to a lower risk of childhood Health's recommendations for folic acid autism, the most severe form of autism. The supplements during pregnancy and emphasise the results show no reduction in the risk of atypical or importance of starting early—preferably before unspecific autism. The study also investigated the conception. prevalence of Asperger syndrome, but the number of examined children was too low to give a reliable Method result. -

UNHCR COVID-19 Global Emergency Response
GLOBAL COVID-19 EMERGENCY RESPONSE 17 February 2021 UNHCR COVID-19 Preparedness and Response Highlights COVID-19 update ■ UNHCR and Gavi, the Vaccine Alliance, signed a Memorandum of Over 46,000 Understanding (MoU) on 03 February 2020, with the overall goal of ensuring reported cases refugees and other forcibly displaced can access vaccines on par with of COVID-19 nationals. The MoU also looks at expanding coverage and quality of among forcibly displaced people immunization services, promoting equity in access and uptake of vaccines, and strengthening health systems at community and primary care level. across 103 countries ■ Jordan has become one of the world’s first countries to start COVID-19 vaccinations for refugees, including vaccinations in Za’atari refugee camp on 15 February. UNHCR has been working with the Jordanian government to increase of vaccinate refugees and provide critical health, sanitation, hygiene and logistical some 7,500 cases compared support. UNHCR appeals to all countries to follow suit and include refugees in to the previous their national vaccination drives in line with COVAX allocation principles. reporting period ■ In 2020, 39.4 million persons of concern received COVID-19 assistance (numbers as of 08 February including access to protection services, shelter, health, and education. This 2021) includes over 8.5 million individuals who received cash assistance. ■ Despite an estimated 1.44 million refugees in urgent need of resettlement globally, less than 23,000 were resettled through UNHCR last year. These are the lowest refugee resettlement numbers UNHCR has witnessed in almost two decades. The drop stems from low quotas put forward by states, as well as the impact of COVID-19, which delayed departures and programmes. -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -

References for L'affaire Wakefield: Shades Of
REFERENCES FOR L’AFFAIRE WAKEFIELD: SHADES OF DREYFUS 1 Alpha-1-Antitrypsin, Autism, And Coeliac Disease, John Walker-Smith and Judith Andrews, The Lancet, 1972; cited by Professor Walker-Smith in his autobiography, Enduring Memories, 2012, p. 211-3; Perinatal Measles Infection And Subsequent Crohn’s Disease. Ekbom A, Wakefield AJ, Zack M, Adami HO. Lancet 1994; Detection Of Immunoreactive Antigen, With A Monoclonal Antibody To Measles Virus, In Tissue From A Patient With Crohn's Disease, Hiroyuki Miyamoto, Tomoyuki Tanaka, Noritoshi Kitamoto, Yoshihiro Fukuda, Takashi Shimoyama, Journal of Gastroenterology, 1995 2 Closed Financial Loops, Kevin De Jesus-Morales and Vinay Prasad , The Hastings Center, 2017 3 Of Measles and Flu, Editor’s Choice, Fiona Godlee, October 2006 4 The Tamiflu Trials, Editorial by Elizabeth Loder, David Tovey, and Fiona Godlee, BMJ, 2014 5 The Next MMR – Could We Do Better? Fiona Godlee, Head of BMJ Knowledge, power point presentation, British National Formulary, 2004 6 Reflections on Investigating Wakefield, Brian Deer, BMJ, February 2, 2010 7 Deposition of Jane Smith, BMJ Deputy Editor June 28, 2012, Wakefield vs. BMJ (Texas litigation) BMJ 8523, p. 45-46 8 How The Case Against The MMR Vaccine Was Fixed, BMJ, January 6, 2011; How The Vaccine Crisis Was Meant To Make Money, BMJ, January 11, 2011; The Lancet’s Two Days To Bury Bad News, BMJ, January 18, 2011 9 Wakefield’s Article Linking MMR Vaccine And Autism Was Fraudulent , Editorial by Fiona Godlee, Editor-in-Chief; Jane Smith, Deputy Editor; Harvey Marcovitz, Associate Editor, BMJ, January 5, 2011 10 CNN, Anderson Cooper 360 Degrees, January 5, 2011 92 11 Merck’s MMR is the only one used in the U.S. -

Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging
International Journal of Environmental Research and Public Health Review Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging Amanda Hudson 1,2,* and William J. Montelpare 3 1 Department of Mental Health and Addiction, Health PEI, Charlottetown, PE C1C 1M3, Canada 2 Department of Health Management, University of Prince Edward Island, Charlottetown, PE C1A 4P3, Canada 3 Department of Applied Human Sciences, University of Prince Edward Island, Charlottetown, PE C1A 4P3, Canada; [email protected] * Correspondence: [email protected] Abstract: Objectives: Successful immunization programs require strategic communication to increase confidence among individuals who are vaccine-hesitant. This paper reviews research on determinants of vaccine hesitancy with the objective of informing public health responses to COVID-19. Method: A literature review was conducted using a broad search strategy. Articles were included if they were published in English and relevant to the topic of demographic and individual factors associated with vaccine hesitancy. Results and Discussion: Demographic determinants of vaccine hesitancy that emerged in the literature review were age, income, educational attainment, health literacy, rurality, and parental status. Individual difference factors included mistrust in authority, disgust sensitivity, and risk aversion. Conclusion: Meeting target immunization rates will require robust public health campaigns that speak to individuals who are vaccine-hesitant in their attitudes and behaviours. Based on the assortment of demographic and individual difference factors that contribute Citation: Hudson, A.; Montelpare, to vaccine hesitancy, public health communications must pursue a range of strategies to increase W.J. Predictors of Vaccine Hesitancy: public confidence in available COVID-19 vaccines. Implications for COVID-19 Public Health Messaging. -

Environmental Health Issue
FIFTH EDITION 2006, Volume 45 R5 Environmental Health and Autism In thIs Issue: Time To GeT a Grip By martha r. Herbert, m.D., ph.D. Beyond Behavior—Biomedical Diagnoses in Autism spectrum Disorders By Margaret L. Bauman, M.D. transforming the Public Debate on neurotoxicants By Elise Miller, M.Ed. ADVERTISEMENT ADVERTISEMENT Autism does not have to be a life sentence You’re not about to give up on your child. Neither Are We. Since , the Autism Treatment Center of America™ has provided innovative training programs for parents and professionals caringifor children challenged by Autism Spectrum Disorders and related developmental difficulties. • Practical Tools • Powerful Results • Limitless Hope c Help your child improve in all areas of over p learning, development, communication and hoto skill acquisition. : © W I Join us for our internationally-acclaimed ll T ERR Son-Rise Program® Start-Up, a y comprehensive weeklong training program for parents and professionals. We don’t put limits on the possibilities for your child. Free 25-Minute Initial Call 877-766-7473 We’ll give you the keys to unlock their world. HOME OF THE SON-RISE PROGRAM® SINCE 1983 South Undermountain Road Sheffield, MA - USA Telephone: -- • E-mail: [email protected] www.autismtreatment.com Copyright © 2006 by The Option Institute & Fellowship. All rights reserved. 02.06-6 CONTENTS December 2006 page 18 SpOTlIGHT Time to Get A Grip By marTHa r. HerBerT, m.D., pH.D. Does an environmental role in autism make sense? How do we decide? And if environment is involved in autism, what do we do about it? These are challenging questions. -

Sensitivity and Specificity of Early Screening for Autism
BJPsych Open (2019) 5, e41, 1–8. doi: 10.1192/bjo.2019.34 Sensitivity and specificity of early screening for autism Pål Surén, Alexandra Saasen-Havdahl, Michaeline Bresnahan, Deborah Hirtz, Mady Hornig, Catherine Lord, Ted Reichborn-Kjennerud, Synnve Schjølberg, Anne-Siri Øyen, Per Magnus, Ezra Susser, W. Ian Lipkin and Camilla Stoltenberg Background (95% CI 58–79) for ASD without phrase speech and 34% (95% CI – Early identification and diagnosis is beneficial for children with 29 40) for ASD with phrase speech. Specificity was then reduced – autism spectrum disorder (ASD). Universal early screening is to 89% (95% CI 89 90). recommended by many experts, but disputed because evidence is limited, and sensitivity and specificity in general populations Conclusions are largely unknown. Early ASD screening with a parent checklist had low sensitivity. It identified mainly individuals with ASD with significant develop- Aims mental delay and captured very few children with ASD with To estimate the sensitivity and specificity of early population- cognitive skills in the normal range. Increasing sensitivity was not based screening for ASDs. possible without severely compromising specificity. Method Declaration of interest The study was based on the Norwegian Mother and Child Cohort C.L. receives royalty for the Social Communication Study. The 36-month cohort questionnaire included the Social Questionnaire, which she has co-authored. Communication Questionnaire (SCQ), a 40-item screening instrument for ASD. Keywords Results Autistic spectrum disorders; developmental disorders; A total of 58 520 mothers (58%) responded to the questionnaire. epidemiology. By the end of follow-up on 31 December 2015, 385 (0.7%) indi- viduals with ASD had been identified among the responders’ Copyright and usage children. -

Prenatal Exposure to Antipsychotic Medication Does Not Increase Odds of Autism, ADHD
Spectrum | Autism Research News https://www.spectrumnews.org NEWS Prenatal exposure to antipsychotic medication does not increase odds of autism, ADHD BY PETER HESS 20 AUGUST 2021 Listen to this story: Children born to mothers who take antipsychotic medications during pregnancy do not have elevated odds of autism or attention deficit hyperactivity disorder (ADHD), nor are they more likely to be born preterm or underweight, according to a study released this past Monday in JAMA Internal Medicine. Some women with schizophrenia, Tourette syndrome or bipolar disorder take antipsychotic drugs, such as aripiprazole, haloperidol or risperidone. Clinicians have long debated whether women should discontinue these medications during pregnancy out of concern for the drugs’ effects on the developing fetus. But children born to mothers who take antipsychotics during pregnancy and to those who do not take them have similar outcomes, the new work shows. “Our findings do not support a recommendation for women to discontinue their regular antipsychotic treatment during pregnancy,” says senior investigator Kenneth Man, research fellow at the University College London School of Pharmacy in the United Kingdom. Prescribing antipsychotics during pregnancy can help prevent potentially dangerous psychotic episodes and ensure that an expectant mother can take care of herself, says Mady Hornig, associate professor of epidemiology at Columbia University, who was not involved in the study. “We certainly don’t want to be cavalier about the use of any medication during pregnancy, but one 1 / 2 Spectrum | Autism Research News https://www.spectrumnews.org also wants to balance out the implications of not treating.” Any apparent effects of antipsychotics on a developing fetus are likely due to the condition being treated, rather than the treatment, the study shows. -
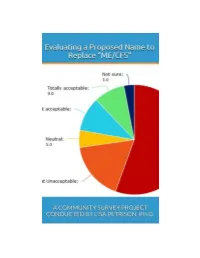
Read the Full Survey Report
Evaluating a Proposed Name to Replace ‘ME/CFS’: A Community Survey Project Conducted by Lisa Petrison, Ph.D. Published by Paradigm Change (March 2015) Copyright 2015 www.paradigmchange.me 2 Table of Contents Executive Summary ...................................................................................................................................... 5 Part 1 – Results Overview .......................................................................................................................... 13 Part 2 - Implications ................................................................................................................................... 29 Part 3 – Survey Questions .......................................................................................................................... 49 Part 4 – Main Survey Results ..................................................................................................................... 66 Part 5 – ME vs. Non-ME Results ............................................................................................................... 110 Part 6 – US ME vs. Non-ME Results ......................................................................................................... 127 Part 7 – Related Illnesses Results ............................................................................................................ 161 Part 8 – US vs. Non-US Results ................................................................................................................. 181 -

Vaccine Adjuvants: from 1920 to 2015 and Beyond
Vaccines 2015, 3, 320-343; doi:10.3390/vaccines3020320 OPEN ACCESS vaccines ISSN 2076-393X www.mdpi.com/journal/vaccines Review Vaccine Adjuvants: from 1920 to 2015 and Beyond Alberta Di Pasquale 1,*, Scott Preiss 1, Fernanda Tavares Da Silva 1 and Nathalie Garçon 2 1 GSK Vaccines, Avenue Fleming, 1300 Wavre, Belgium; E-Mails: [email protected] (S.P.); [email protected] (F.T.D.S.) 2 Bioaster, 321 Avenue Jean Jaurès, 6700 Lyon, France; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-10-85-3573. Academic Editor: Diane M. Harper Received: 23 February 2015 / Accepted: 9 April 2015 / Published: 16 April 2015 Abstract: The concept of stimulating the body’s immune response is the basis underlying vaccination. Vaccines act by initiating the innate immune response and activating antigen presenting cells (APCs), thereby inducing a protective adaptive immune response to a pathogen antigen. Adjuvants are substances added to vaccines to enhance the immunogenicity of highly purified antigens that have insufficient immunostimulatory capabilities, and have been used in human vaccines for more than 90 years. While early adjuvants (aluminum, oil-in-water emulsions) were used empirically, rapidly increasing knowledge on how the immune system interacts with pathogens means that there is increased understanding of the role of adjuvants and how the formulation of modern vaccines can be better tailored towards the desired clinical benefit. Continuing safety evaluation of licensed vaccines containing adjuvants/adjuvant systems suggests that their individual benefit-risk profile remains favorable.