Vaccine Adjuvants: from 1920 to 2015 and Beyond
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Program Evaluation Key Outcomes and Addressing Remaining
Vaccine 31S (2013) J73–J78 Contents lists available at ScienceDirect Vaccine jou rnal homepage: www.elsevier.com/locate/vaccine Key outcomes and addressing remaining challenges—Perspectives from a final ଝ evaluation of the China GAVI project a,1 a,1 a,1 a,1 b c Weizhong Yang , Xiaofeng Liang , Fuqiang Cui , Li Li , Stephen C. Hadler , Yvan J. Hutin , d a,∗ Mark Kane , Yu Wang a Chinese Center for Disease Control and Prevention, Beijing, China b Centers for Disease Control and Prevention, Atlanta, USA c Europe Center for Disease Control and Prevention, Stockholm, Sweden d Mercer Island, Washington, USA a r t a b i c s t l r e i n f o a c t Article history: During the China GAVI project, implemented between 2002 and 2010, more than 25 million children Received 6 June 2012 received hepatitis B vaccine with the support of project, and the vaccine proved to be safe and effective. Received in revised form 24 July 2012 With careful consideration for project savings, China and GAVI continually adjusted the budget, addi- Accepted 24 September 2012 tionally allowing the project to spend operational funds to support demonstration projects to improve timely birth dose (TBD), conduct training of EPI staff, and to monitor the project impact. Results from the final evaluation indicated the achievement of key outcomes. As a result of government co-investment, Keywords: human resources at county level engaged in hepatitis B vaccination increased from 29 per county on GAVI Project average in 2002 to 66 in 2009. All project counties funded by the GAVI project use auto-disable syringes Outcomes for hepatitis B vaccination and other vaccines. -

Information and Communication Guide on Vaccines
Module 2 EUROPEAN NURSE Information and Communication Guide ON VACCINES The European Specialist Nurses Organisation (ESNO) is a non-profit organisation with the goal to facilitate and provide an effective framework for communication and co-operation between the European Specialist Nurses Organisations and its constituent members. ESNO represents the mutual interests and benefits of these organisations to the wider European community in the interest of the public health. Members of ESNO consist of individual European specialist nurses member organizations and associates, both institutional and individual. The organisation focusses on enhancing the capacity and capability of specialists nurses to deliver hight quality healthcare by raising and harmonise specialist nursing education standards and actively contribute to health themes and threats, providing the best possible expertise, both national and in European cross border context. A publication from the European Specialist Nurses Organisation April 2021 - www.esno.org Copyright: ©2021 European Specialist Nurses Organisation. All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the publisher, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law. CONTENTS Vaccination 1 Introduction 5 2 Principles of vaccination 6 2.1 Principle -

Vaccines 101 the Very Last Day That I Was a Pediatric Resident. Um, Many
Vaccines 101 The very last day that I was a pediatric resident. Um, many years ago, a toddler walked into the emergency room and uh, and progressively got sicker and sicker. That's Dr. Katherine Edwards, a world expert in pediatric infectious disease in vaccinology. She's also a professor of pediatrics at Vanderbilt university and she's been working on vaccines for 40 years. I did a spinal tap on her and realized that she had Haemophilus influenza, typ e B meningitis, Haemophilus influenza, type B or HIB is it bacteria normally found in our nose and throat that can lead to very serious life threatening infections and no matter what I did in that day and into the night in terms of prompt antibiotics and she'd just been sick a few hours and, and fluids and all the, you know, ventilators and all the best things that modern medicine, she died, the vaccine for hip was not available until the 1990s. And until it did become available, hip disease affected approximately 25,000 children each year with things like meningitis, pneumonia, and bloodstream infections. And at that time in the hospital that I was practicing at any one time, there were generally five or six patients that had Haemophilus meningitis or invasive disease or some complication of this particular infection. And we knew that from the basic science that if you had antibody to the capsule or to the coat of the organism, that you were protected from disease. But we really didn't know how to make little kids make antibody. -

Bcch 2019-20 Flu Vaccine – Oncology Clinic
BCCH 2019-20 FLU VACCINE – ONCOLOGY CLINIC We are providing influenza vaccines for patients who are on therapy and have platelets > 50,000 and ANC over 0.5 (and expected to stay above 0.5 for the next 48 hours, in order to minimize risk of admission in case of fever). Please do not schedule appointments for flu shots only. Please advise the family that immunization of all family members is recommended. The Oncology Outpatient Clinic is not providing immunizations for family members or for those patients who are OFF therapy. Flu shots can be given by family doctors to patients and their families, or they can get a flu shot at the BCCH drop-in Family Immunization clinic in the Ambulatory Care Building, across from the Ambulatory Care Pharmacy on week days in the fall months. Pediatric oncology patients can also get a flu shot at almost any community pharmacy at no cost as they are considered “at risk.” Age Dose** Doses required 6 months – 9 years 0.5 mL IM 1 or 2* > 9 years 0.5 mL IM 1 Less than 6 months Not recommended *Two doses administered at least 4 weeks apart are recommended for children under 9 years of age who are receiving influenza vaccine for the first time. **The recommended site of vaccination is the deltoid muscle for adults and older children. The preferred site for infants and young children is the anterolateral aspect of the thigh. Contraindications: • Febrile illness • Thimerosal sensitivity Special considerations: • Egg allergic individuals (including those who have experienced anaphylaxis following egg ingestion) can be immunized with inactivated influenza vaccine (such as Flulaval tetra) • Thimerosal allergic patients should be vaccinated using Agriflu which can be obtained from local public health units, (but not in the oncology clinic). -

HIV Vaccine Development Dr. Patricia Fast 1
HIV Vaccine Development Dr. Patricia Fast HIV Vaccine Development Dr. Patricia Fast (MD, PhD) Senior Technical Advisor, International AIDS Vaccine Initiative Adjunct Clinical Associate Professor, Infectious Disease, Pediatrics Stanford University School of Medicine How It Began 1984 • Human Immunodeficiency Virus (HIV-1) discovered as the cause of AIDS • Prediction: a vaccine will soon be developed! Non-Human Primate Models Focusing on this dominant model • Simian Immunodeficiency Virus (SIV) lead to a bias – SIV Mac 239 causes AIDS-like disease in Macaques almost impossible to neutralize • SHIV hybrid (with HIV Envelope) allows research into neutralizing antibodies in macaques • Chimpanzees can be infected by HIV, but seldom get AIDS False starts • Traditional vaccine approaches fail in NHP model . Killed SIV does not really protect against SIV o Initial positive result was an artifact . Live attenuated SIV protects, but is not safe o Attenuated vaccine was shown to regain virulence The screen versions of these slides have full details of copyright and acknowledgements 1 HIV Vaccine Development Dr. Patricia Fast How would an AIDS vaccine work? A note about virus biology • HIV has many mechanisms to escape immune recognition e.g. : . Rapid formation of variation . Structural aspects How would an AIDS vaccine work? Immune Mechanisms • T cells . Kill virus-infected cells . Slow down or stop replication of viruses within cells . Can directly kill virus infected cells . Secrete substances that block viral replication Can neutralize virus when they prevent entry; by binding viral envelope protein or the cellular receptors HIV is extremely variable Europe and North America East Africa Southern Africa, India and China Part of an HIV phylogenetic tree The screen versions of these slides have full details of copyright and acknowledgements 2 HIV Vaccine Development Dr. -

UNHCR COVID-19 Global Emergency Response
GLOBAL COVID-19 EMERGENCY RESPONSE 17 February 2021 UNHCR COVID-19 Preparedness and Response Highlights COVID-19 update ■ UNHCR and Gavi, the Vaccine Alliance, signed a Memorandum of Over 46,000 Understanding (MoU) on 03 February 2020, with the overall goal of ensuring reported cases refugees and other forcibly displaced can access vaccines on par with of COVID-19 nationals. The MoU also looks at expanding coverage and quality of among forcibly displaced people immunization services, promoting equity in access and uptake of vaccines, and strengthening health systems at community and primary care level. across 103 countries ■ Jordan has become one of the world’s first countries to start COVID-19 vaccinations for refugees, including vaccinations in Za’atari refugee camp on 15 February. UNHCR has been working with the Jordanian government to increase of vaccinate refugees and provide critical health, sanitation, hygiene and logistical some 7,500 cases compared support. UNHCR appeals to all countries to follow suit and include refugees in to the previous their national vaccination drives in line with COVAX allocation principles. reporting period ■ In 2020, 39.4 million persons of concern received COVID-19 assistance (numbers as of 08 February including access to protection services, shelter, health, and education. This 2021) includes over 8.5 million individuals who received cash assistance. ■ Despite an estimated 1.44 million refugees in urgent need of resettlement globally, less than 23,000 were resettled through UNHCR last year. These are the lowest refugee resettlement numbers UNHCR has witnessed in almost two decades. The drop stems from low quotas put forward by states, as well as the impact of COVID-19, which delayed departures and programmes. -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Humanized Mice for Live-Attenuated Vaccine Research: from Unmet Potential to New Promises
Review Humanized Mice for Live-Attenuated Vaccine Research: From Unmet Potential to New Promises Aoife K. O’Connell and Florian Douam * Department of Microbiology, National Emerging Infectious Diseases Laboratories, Boston University School of Medicine, Boston, MA 02118, USA; [email protected] * Correspondence: [email protected] Received: 21 December 2019; Accepted: 13 January 2020; Published: 21 January 2020 Abstract: Live-attenuated vaccines (LAV) represent one of the most important medical innovations in human history. In the past three centuries, LAV have saved hundreds of millions of lives, and will continue to do so for many decades to come. Interestingly, the most successful LAVs, such as the smallpox vaccine, the measles vaccine, and the yellow fever vaccine, have been isolated and/or developed in a purely empirical manner without any understanding of the immunological mechanisms they trigger. Today, the mechanisms governing potent LAV immunogenicity and long-term induced protective immunity continue to be elusive, and therefore hamper the rational design of innovative vaccine strategies. A serious roadblock to understanding LAV-induced immunity has been the lack of suitable and cost-effective animal models that can accurately mimic human immune responses. In the last two decades, human-immune system mice (HIS mice), i.e., mice engrafted with components of the human immune system, have been instrumental in investigating the life-cycle and immune responses to multiple human-tropic pathogens. However, their use in LAV research has remained limited. Here, we discuss the strong potential of LAVs as tools to enhance our understanding of human immunity and review the past, current and future contributions of HIS mice to this endeavor. -

Influenza Whoinsert Generic Name As on 080710
capacity. Healthcare providers need to assess the benefit and potential risks of administering the vaccine to pregnant women. It is not known whether Influenza Vaccine(Human,Live Attenuated) is excreted in human milk. Therefore, as some viruses are Influenza Vaccine excreted in human milk and additionally, because of the possibility of shedding of vaccine virus and the close proximity of a nursing infant and mother, caution should be exercised if Influenza Vaccine(Human,Live Attenuated) is administered to nursing Sii mothers. (Human, Live Attenuated) Effects on ability to drive and use machines The vaccine is unlikely to produce an effect on the ability to drive and use machines. Pandemic (H1N1) (Freeze-Dried) ADVERSE REACTIONS In clinical trials a few local and systemic reaction were observed. They were mild to moderate in severity and resolved without DESCRIPTION any sequelae. Influenza Vaccine(Human,Live Attenuated) Pandemic (H1N1), freeze dried is a live monovalent vaccine for administration by Local : Nasal discomfort, stuffy nose, sneezing, runny nose, loss of smell red eyes, lacrimation, facial swelling. intranasal spray. The influenza vaccine contains Influenza virus cultivated on embryonated eggs. Systemic : Headache, fatigue, myalgia, arthralgia, irritability, loss of appetite, sore throat, cough, diarrhoea. The incidence was similar in both the study groups. COMPOSITION There were a few unsolicited event reported in both the groups and none of them were causally related to study vaccines. [Propagated in Embryonated hen eggs (SPF)] Each single dose of 0.5 ml contains: 7 OVERDOSE A/17/California/2009/38 > 10 EID50 No case of overdose has been reported. Gelatin (Partially hydrolyzed) 2.5%, Sorbitol 5%, L-Alanine 0.1% L-Histidine 0.21%, Tricine 0.3%, L-Arginine hydrochloride 1.6% Lactalbumin hydrolysate 0.35%, Phosphate buffer saline Base PHARMACOLOGICAL PROPERTIES Reconstitute with Sterile Water for Inhalation USP. -

An Oral Live Attenuated Vaccine Strategy Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2/2019-Ncov)
Research Ideas and Outcomes 6: e53767 doi: 10.3897/rio.6.e53767 Research Idea An oral live attenuated vaccine strategy against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2/2019-nCoV) Madhusudana Girija Sanal‡, Ravi Chandra Dubey§ ‡ Institute of Liver and Biliary Sciences, New Delhi, India § South Asian University, New Delhi, India Corresponding author: Madhusudana Girija Sanal ([email protected]) Reviewed v1 Received: 29 Apr 2020 | Published: 13 May 2020 Citation: Sanal MG, Dubey RC (2020) An oral live attenuated vaccine strategy against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2/2019-nCoV). Research Ideas and Outcomes 6: e53767. https://doi.org/10.3897/rio.6.e53767 Abstract Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2/2019-nCoV) infection has become a pandemic called COVID-19. The virus binds to angiotensin converting enzyme 2 (ACE2) and TMPRSS2 which are abundantly expressed on various human cells including lung epithelial cells and intestinal cells and the virus can infect these cells. Currently no specific treatments or vaccines are available for this disease. A per oral live attenuated vaccine can be a good strategy in SARS-CoV-2 infection because the attenuated virus initially infects the gut, stimulates the mucosa associated immune system sparing the respiratory system during the initial immune response. The live virus can also spread in the community boosting herd immunity. Keywords Oral Live Attenuated Vaccine, Severe Acute Respiratory Syndrome Coronavirus 2/SARS- CoV-2/2019-nCoV, Angiotensin Converting Enzyme-2 (ACE2), Gut Infection, Proximal and Distal Enterocytes, Herd Immunity © Sanal M, Dubey R. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -
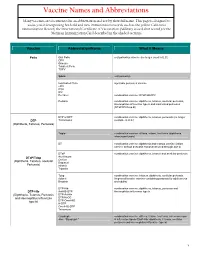
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus,