Center for Solutions for ME/CFS W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Controversial CFS Researcher Arrested and Jailed - Scienceinsider
Controversial CFS Researcher Arrested and Jailed - ScienceInsider http://news.sciencemag.org/scienceinsider/2011/11/controversial-cfs-researcher-arr.html?ref=em... AAAS.ORG FEEDBACK HELP LIBRARIANS Daily News Enter Search Term ADVANCED ALERTS ACCESS RIGHTS MY ACCOUNT SIGN IN News Home ScienceNOW ScienceInsider Premium Content from Science About Science News Home > News > ScienceInsider > November 2011 > Controversial CFS Researcher Arrested and Jailed Controversial CFS Researcher Arrested and Jailed by Jon Cohen on 19 November 2011, 6:46 PM | 16 Comments Email Print | 0 More PREVIOUS ARTICLE NEXT ARTICLE 1 of 5 12/1/11 10:06 AM Controversial CFS Researcher Arrested and Jailed - ScienceInsider http://news.sciencemag.org/scienceinsider/2011/11/controversial-cfs-researcher-arr.html?ref=em... Judy Mikovits, who has been in the spotlight for the past 2 years after Science published a controversial report by her group that tied a novel mouse retrovirus to chronic fatigue syndrome (CFS), is now behind bars. Sheriffs in Ventura County, California, arrested Mikovits yesterday on felony charges that she is a fugitive from justice. She is being held at the Todd Road Jail in Santa Paula without bail. But ScienceInsider could obtain only sketchy details about the specific charges against her. The Ventura County sheriff's office told ScienceInsider that it had no available details about the charges and was acting upon a warrant issued by Washoe County in Nevada. A spokesperson for the Washoe County Sheriff's Office told ScienceInsider that it did not issue the warrant, nor did the Reno or Sparks police department. He said it could be from one of several federal agencies in Washoe County. -

Ketogenic Diet and Immunotherapy in Cancer and Neurological Diseases
American Journal of Immunology Editorials Ketogenic Diet and Immunotherapy in Cancer and Neurological Diseases. 4th International Congress on Integrative Medicine, 1, 2 April 2017, Fulda, Germany 1Thomas Peter and 2Marco Ruggiero 1Therapy Center Anuvindati, Bensheim, Germany 2Silver Spring Sagl, Arzo-Mendrisio, Switzerland Article history Abstract: In this Meeting Report, we review proceedings and comment Received: 23-08-2017 on talks given at the 4 th International Congress on Integrative Medicine Revised: 23-08-2017 Accepted: 25-09-2017 held on April 1 and 2, 2017, in Fulda, Germany. The Theme this year was “Ketogenic Diet and Immunotherapy in Cancer and Neurological Diseases.” Corresponding Author: Speakers came from Europe and the United States and the Congress was Marco Ruggiero attended by more than 100 interested parties from all over the world. Silver Spring Sagl. Via Raimondo Rossi 24, Arzo- Mendrisio 6864, Switzerland Keywords: Ketogenic Diet, Immunotherapy, Integrative Medicine, Email: [email protected] Cancer, Autism, Lyme Disease Introduction a talk entitled "Exploiting cancer metabolism with th ketosis and hyperbaric oxygen". Dr. Poff lectured on the The 4 International Congress on Integrative synergism between nutritional ketosis and hyperbaric Medicine, was held on 1 and 2 April, 2017, in Fulda, oxygen in reducing tumor burden in experimental Germany. The theme of this Congress was "Ketogenic animals and offered insights on the mechanism of action Diet and Immunotherapy in Cancer and Neurological of ketone bodies in a variety of conditions from seizures Diseases" (Ketogene Ernährung und Immuntherapie bei to cancer proliferation. The role of exogenous ketone Krebs und Neurologischen Erkrankungen). bodies in counteracting cancer cell proliferation even in The Congress brought together researchers from all the absence of carbohydrate restriction was also over the world to share their views and research on the discussed. -

Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children
ORIGINAL CONTRIBUTION Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children ˚ ´ Pal Suren, MD, MPH Importance Prenatal folic acid supplements reduce the risk of neural tube defects Christine Roth, MSc in children, but it has not been determined whether they protect against other neu- Michaeline Bresnahan, PhD rodevelopmental disorders. Margaretha Haugen, PhD Objective To examine the association between maternal use of prenatal folic acid supplements and subsequent risk of autism spectrum disorders (ASDs) (autistic disor- Mady Hornig, MD der, Asperger syndrome, pervasive developmental disorder–not otherwise specified Deborah Hirtz, MD [PDD-NOS]) in children. Kari Kveim Lie, MD Design, Setting, and Patients The study sample of 85 176 children was derived from the population-based, prospective Norwegian Mother and Child Cohort Study W. Ian Lipkin, MD (MoBa). The children were born in 2002-2008; by the end of follow-up on March 31, Per Magnus, MD, PhD 2012, the age range was 3.3 through 10.2 years (mean, 6.4 years). The exposure of Ted Reichborn-Kjennerud, MD, PhD primary interest was use of folic acid from 4 weeks before to 8 weeks after the start of pregnancy, defined as the first day of the last menstrual period before conception. Synnve Schjølberg, MSc Relative risks of ASDs were estimated by odds ratios (ORs) with 95% CIs in a logistic George Davey Smith, MD, DSc regression analysis. Analyses were adjusted for maternal education level, year of birth, and parity. Anne-Siri Øyen, PhD Main Outcome Measure Specialist-confirmed diagnosis of ASDs. Ezra Susser, MD, DrPH Results At the end of follow-up, 270 children in the study sample had been diag- Camilla Stoltenberg, MD, PhD nosed with ASDs: 114 with autistic disorder, 56 with Asperger syndrome, and 100 with PDD-NOS. -

Lower Autism Risk with Folic Acid Supplements in Pregnancy 12 February 2013
Lower autism risk with folic acid supplements in pregnancy 12 February 2013 Women who took folic acid supplements in early of folic acid supplements in the mother during pregnancy almost halved the risk of having a child pregnancy and a reduced risk of childhood autism. with autism. Beginning to take folic acid supplements later in pregnancy did not reduce the "The study does not prove that folic acid risk. This is shown in new findings from the ABC supplements can prevent childhood autism. Study and Norwegian Mother and Child Cohort However, the findings are so apparent that they Study published in the Journal of The American constitute a good argument to further examine Medical Association (JAMA). possible causal mechanisms. It should also be ascertained whether folic acid is associated with a Women who took folic acid supplements from four reduced risk of other brain disorders in children," weeks before conception to eight weeks into says Surén. pregnancy had a 40 per cent lower risk of giving birth to children with childhood autism (classic Emphasises the importance of folic acid autism). Use of folic acid supplements midway supplements through pregnancy (week 22) had no effect. The results support the Norwegian Directorate of The findings only apply to a lower risk of childhood Health's recommendations for folic acid autism, the most severe form of autism. The supplements during pregnancy and emphasise the results show no reduction in the risk of atypical or importance of starting early—preferably before unspecific autism. The study also investigated the conception. prevalence of Asperger syndrome, but the number of examined children was too low to give a reliable Method result. -

Aow 1920 37 Coronavirus Infodemic
1. Mark your confusion. 2. Show evidence of a close reading. 3. Write a 1+ page reflection. The Coronavirus Infodemic How the coronavirus sparked a tsunami of misinformation. Here's everything you need to know. Source: TheWeek.com, May 17, 2020 What's being said? Social media has been awash in lies, rumors, and distortions about the coronavirus, its origins, and potential cures almost since the pandemic's start. NewsGuard, a private company that rates websites' reliability, has identified 203 websites throughout the world promoting COVID-19 misinformation. Among the most popular COVID-19 myths were "Garlic can cure COVID-19," "A group funded by Bill Gates patented the coronavirus," and "The COVID-19 virus is a man- made bioweapon." The World Health Organization is calling the deluge of misinformation "an infodemic," while Google, Facebook, and Twitter are instituting policies to help slow the spread of lies. CEO Mark Zuckerberg's Facebook fact-checkers have "taken down hundreds of thousands of pieces of misinformation," and slapped warnings on 40 million posts during March alone, the company says. Last week, YouTube removed a video titled "Plandemic" that had been viewed millions of times, featuring discredited microbiologist and anti-vaxxer Judy Mikovits. In the video and a book that reached No. 1 on Amazon, Mikovits contends the coronavirus was created and spread by a conspiracy of wealthy people and scientists such as Dr. Anthony Fauci, head of the National Institute of Allergy and Infectious Diseases, to bolster vaccine rates and make money. Mikovits has been claiming her career was destroyed by Fauci and other scientists since she falsely reported in a 2009 study, later retracted by the journal that published it, that a retrovirus caused chronic fatigue syndrome. -

SCR-71 Submitted On: 3/18/2021 10:06:08 PM Testimony for PSM on 3/23/2021 1:10:00 PM
SCR-71 Submitted on: 3/18/2021 10:06:08 PM Testimony for PSM on 3/23/2021 1:10:00 PM Testifier Present at Submitted By Organization Position Hearing Isabel Espiritu Individual Oppose No Comments: Aloha, We the people of Hawaii do not want Resolution SCR71 to pass. We should be able to fly without showing any proof of any medical records. This is medical discrimination, unethical and a direct violation on our constitutional rights. SCR-71 Submitted on: 3/22/2021 4:12:11 AM Testimony for PSM on 3/23/2021 1:10:00 PM Testifier Present at Submitted By Organization Position Hearing Lily Gavri Individual Oppose No Comments: Everything that is going on with Covid as well as the entire medical and pharmaceutical industry is built on the germ theory of disease by Louis Pasteur which proposes that particles in the air are the cause of disease. This has never been proven in accordance with Koche’s postulates which are the principles by which microorganisms are scientifically identified and isolated. They are: 1. The microorganism must be identified in all individuals affected by the disease, but not in healthy individuals. 2. The microorganism can be isolated from the diseased individual and grown in culture. 3. When introduced into a healthy individual, the cultured microorganism should cause disease. 4. The microorganism must then be re-isolated from the experimental host, and found to be identical to the original microorganism. This has never been done with a virus. What they do when trying to identify a virus is they take a healthy cell, starve and poison it and when it dies they claim the leftover particles of the dead cell are newly formed “viruses” “The CDC has NEVER isolated and purified ANY alleged "virus." They simply isolate certain particles that are a natural part of the human body's processes and place that isolated particle into a petri dish wherein they mix it with monkey kidney cells and chemicals and starve the cell whereby it begins to break down. -

Dissemination of Vaccine Misinformation on Twitter and Its Countermeasures
Dissertation Dissemination of Vaccine Misinformation on Twitter and Its Countermeasures Christine Chen This document was submitted as a dissertation in March 2021 in partial fulfillment of the requirements of the doctoral degree in public policy analysis at the Pardee RAND Graduate School. The faculty committee that supervised and approved the dissertation consisted of Luke Matthews (Chair), Sarah Nowak and Jeremy Miles. The external reader was Jennifer Golbeck. This dissertation was generously supported by the Anne and James Rothenberg Dissertation Award. PARDEE RAND GRADUATE SCHOOL For more information on this publication, visit http://www.rand.org/pubs/rgs_dissertations/RGSDA1332-1.html Published 2021 by the RAND Corporation, Santa Monica, Calif. is a registered trademarK Limited Print and Electronic Distribution Rights This document and trademarK(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is reQuired from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions.html. The RAND Corporation is a research organization that develops solutions to public policy challenges to help maKe communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND MaKe a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Abstract Outbreaks of vaccine preventable diseases have continued to affect many parts of the United States. -

Environmental Health Issue
FIFTH EDITION 2006, Volume 45 R5 Environmental Health and Autism In thIs Issue: Time To GeT a Grip By martha r. Herbert, m.D., ph.D. Beyond Behavior—Biomedical Diagnoses in Autism spectrum Disorders By Margaret L. Bauman, M.D. transforming the Public Debate on neurotoxicants By Elise Miller, M.Ed. ADVERTISEMENT ADVERTISEMENT Autism does not have to be a life sentence You’re not about to give up on your child. Neither Are We. Since , the Autism Treatment Center of America™ has provided innovative training programs for parents and professionals caringifor children challenged by Autism Spectrum Disorders and related developmental difficulties. • Practical Tools • Powerful Results • Limitless Hope c Help your child improve in all areas of over p learning, development, communication and hoto skill acquisition. : © W I Join us for our internationally-acclaimed ll T ERR Son-Rise Program® Start-Up, a y comprehensive weeklong training program for parents and professionals. We don’t put limits on the possibilities for your child. Free 25-Minute Initial Call 877-766-7473 We’ll give you the keys to unlock their world. HOME OF THE SON-RISE PROGRAM® SINCE 1983 South Undermountain Road Sheffield, MA - USA Telephone: -- • E-mail: [email protected] www.autismtreatment.com Copyright © 2006 by The Option Institute & Fellowship. All rights reserved. 02.06-6 CONTENTS December 2006 page 18 SpOTlIGHT Time to Get A Grip By marTHa r. HerBerT, m.D., pH.D. Does an environmental role in autism make sense? How do we decide? And if environment is involved in autism, what do we do about it? These are challenging questions. -

Sensitivity and Specificity of Early Screening for Autism
BJPsych Open (2019) 5, e41, 1–8. doi: 10.1192/bjo.2019.34 Sensitivity and specificity of early screening for autism Pål Surén, Alexandra Saasen-Havdahl, Michaeline Bresnahan, Deborah Hirtz, Mady Hornig, Catherine Lord, Ted Reichborn-Kjennerud, Synnve Schjølberg, Anne-Siri Øyen, Per Magnus, Ezra Susser, W. Ian Lipkin and Camilla Stoltenberg Background (95% CI 58–79) for ASD without phrase speech and 34% (95% CI – Early identification and diagnosis is beneficial for children with 29 40) for ASD with phrase speech. Specificity was then reduced – autism spectrum disorder (ASD). Universal early screening is to 89% (95% CI 89 90). recommended by many experts, but disputed because evidence is limited, and sensitivity and specificity in general populations Conclusions are largely unknown. Early ASD screening with a parent checklist had low sensitivity. It identified mainly individuals with ASD with significant develop- Aims mental delay and captured very few children with ASD with To estimate the sensitivity and specificity of early population- cognitive skills in the normal range. Increasing sensitivity was not based screening for ASDs. possible without severely compromising specificity. Method Declaration of interest The study was based on the Norwegian Mother and Child Cohort C.L. receives royalty for the Social Communication Study. The 36-month cohort questionnaire included the Social Questionnaire, which she has co-authored. Communication Questionnaire (SCQ), a 40-item screening instrument for ASD. Keywords Results Autistic spectrum disorders; developmental disorders; A total of 58 520 mothers (58%) responded to the questionnaire. epidemiology. By the end of follow-up on 31 December 2015, 385 (0.7%) indi- viduals with ASD had been identified among the responders’ Copyright and usage children. -

Prenatal Exposure to Antipsychotic Medication Does Not Increase Odds of Autism, ADHD
Spectrum | Autism Research News https://www.spectrumnews.org NEWS Prenatal exposure to antipsychotic medication does not increase odds of autism, ADHD BY PETER HESS 20 AUGUST 2021 Listen to this story: Children born to mothers who take antipsychotic medications during pregnancy do not have elevated odds of autism or attention deficit hyperactivity disorder (ADHD), nor are they more likely to be born preterm or underweight, according to a study released this past Monday in JAMA Internal Medicine. Some women with schizophrenia, Tourette syndrome or bipolar disorder take antipsychotic drugs, such as aripiprazole, haloperidol or risperidone. Clinicians have long debated whether women should discontinue these medications during pregnancy out of concern for the drugs’ effects on the developing fetus. But children born to mothers who take antipsychotics during pregnancy and to those who do not take them have similar outcomes, the new work shows. “Our findings do not support a recommendation for women to discontinue their regular antipsychotic treatment during pregnancy,” says senior investigator Kenneth Man, research fellow at the University College London School of Pharmacy in the United Kingdom. Prescribing antipsychotics during pregnancy can help prevent potentially dangerous psychotic episodes and ensure that an expectant mother can take care of herself, says Mady Hornig, associate professor of epidemiology at Columbia University, who was not involved in the study. “We certainly don’t want to be cavalier about the use of any medication during pregnancy, but one 1 / 2 Spectrum | Autism Research News https://www.spectrumnews.org also wants to balance out the implications of not treating.” Any apparent effects of antipsychotics on a developing fetus are likely due to the condition being treated, rather than the treatment, the study shows. -

Vaccines and ME
Vaccines and M.E. – something to consider for the Covid 19 vaccines February 2021 Vaccines in general Many M.E. sufferers will be wondering whether to have the Covid 19 vaccinations; whilst I have no medical qualifications, I would like nevertheless to share some reflections on both general vaccines and information so far gathered specifically on the Covid 19 vaccinations. The effects of vaccines are always risky for M.E. sufferers due to an abnormally functioning immune system in the first placei. For some sufferers their M.E. was triggered by a vaccine: this happened tragically to Lynn Gilderdale aged 14 after the school BCG vaccination.ii I personally remember suffering a relapse of M.E. when I was 16 after receiving the BCG vaccination at school, back in 1980. There have been enough reports of M.E. being triggered by vaccines to prompt a patient survey by the M.E. Association in 2010, with Hepatitis B being reported as the main vaccine trigger for M.E. iii In the USA, a court of law in 2013 ruled that a Hepatitis B vaccine caused a person’s Chronic Fatigue Syndrome. iv Quote by Dr. Charles Shepherd: ‘Anecdotal evidence indicates that a number of vaccinations are occasionally capable of either triggering ME/CFS, or causing an exacerbation of pre-existing symptoms, and the UK CMO Working Group report acknowledged (in section 3.3.2) that vaccinations can occasionally act as a trigger factor in the development of ME/CFS.’v Some of you may have heard of the short film Plandemic where Dr. -
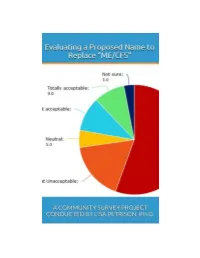
Read the Full Survey Report
Evaluating a Proposed Name to Replace ‘ME/CFS’: A Community Survey Project Conducted by Lisa Petrison, Ph.D. Published by Paradigm Change (March 2015) Copyright 2015 www.paradigmchange.me 2 Table of Contents Executive Summary ...................................................................................................................................... 5 Part 1 – Results Overview .......................................................................................................................... 13 Part 2 - Implications ................................................................................................................................... 29 Part 3 – Survey Questions .......................................................................................................................... 49 Part 4 – Main Survey Results ..................................................................................................................... 66 Part 5 – ME vs. Non-ME Results ............................................................................................................... 110 Part 6 – US ME vs. Non-ME Results ......................................................................................................... 127 Part 7 – Related Illnesses Results ............................................................................................................ 161 Part 8 – US vs. Non-US Results ................................................................................................................. 181