The Kobresia Pygmaea Ecosystem of the Tibetan Highlands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecosystem Profile Madagascar and Indian
ECOSYSTEM PROFILE MADAGASCAR AND INDIAN OCEAN ISLANDS FINAL VERSION DECEMBER 2014 This version of the Ecosystem Profile, based on the draft approved by the Donor Council of CEPF was finalized in December 2014 to include clearer maps and correct minor errors in Chapter 12 and Annexes Page i Prepared by: Conservation International - Madagascar Under the supervision of: Pierre Carret (CEPF) With technical support from: Moore Center for Science and Oceans - Conservation International Missouri Botanical Garden And support from the Regional Advisory Committee Léon Rajaobelina, Conservation International - Madagascar Richard Hughes, WWF – Western Indian Ocean Edmond Roger, Université d‘Antananarivo, Département de Biologie et Ecologie Végétales Christopher Holmes, WCS – Wildlife Conservation Society Steve Goodman, Vahatra Will Turner, Moore Center for Science and Oceans, Conservation International Ali Mohamed Soilihi, Point focal du FEM, Comores Xavier Luc Duval, Point focal du FEM, Maurice Maurice Loustau-Lalanne, Point focal du FEM, Seychelles Edmée Ralalaharisoa, Point focal du FEM, Madagascar Vikash Tatayah, Mauritian Wildlife Foundation Nirmal Jivan Shah, Nature Seychelles Andry Ralamboson Andriamanga, Alliance Voahary Gasy Idaroussi Hamadi, CNDD- Comores Luc Gigord - Conservatoire botanique du Mascarin, Réunion Claude-Anne Gauthier, Muséum National d‘Histoire Naturelle, Paris Jean-Paul Gaudechoux, Commission de l‘Océan Indien Drafted by the Ecosystem Profiling Team: Pierre Carret (CEPF) Harison Rabarison, Nirhy Rabibisoa, Setra Andriamanaitra, -

AP ART HISTORY--Unit 5 Study Guide (Non Western Art Or Art Beyond the European Tradition) Ms
AP ART HISTORY--Unit 5 Study Guide (Non Western Art or Art Beyond the European Tradition) Ms. Kraft BUDDHISM Important Chronology in the Study of Buddhism Gautama Sakyamuni born ca. 567 BCE Buddhism germinates in the Ganges Valley ca. 487-275 BCE Reign of King Ashoka ca. 272-232 BCE First Indian Buddha image ca. 1-200 CE First known Chinese Buddha sculpture 338 CE First known So. China Buddha image 437 CE The Four Noble Truthsi 1. Life means suffering. Life is full of suffering, full of sickness and unhappiness. Although there are passing pleasures, they vanish in time. 2. The origin of suffering is attachment. People suffer for one simple reason: they desire things. It is greed and self-centeredness that bring about suffering. Desire is never satisfied. 3. The cessation of suffering is attainable. It is possible to end suffering if one is aware of his or her own desires and puts and end to them. This awareness will open the door to lasting peace. 4. The path to the cessation of suffering. By changing one’s thinking and behavior, a new awakening can be reached by following the Eightfold Path. Holy Eightfold Pathii The principles are • Right Understanding • Right Intention • Right Speech • Right Action • Right Livelihood • Right Effort • Right Awareness • Right Concentration Following the Eightfold Path will lead to Nirvana or salvation from the cycle of rebirth. Iconography of the Buddhaiii The image of the Buddha is distinguished in various different ways. The Buddha is usually shown in a stylized pose or asana. Also important are the 32 lakshanas or special bodily features or birthmarks. -

Major Geographic Qualities of South Asia
South Asia Chapter 8, Part 1: pages 402 - 417 Teacher Notes DEFINING THE REALM I. Major geographic qualities of South Asia - Well defined physiography – bordered by mountains, deserts, oceans - Rivers – The Ganges supports most of population for over 10,000 years - World’s 2nd largest population cluster on 3% land mass - Current birth rate will make it 1st in population in a decade - Low income economies, inadequate nutrition and poor health - British imprint is strong – see borders and culture - Monsoons, this cycle sustains life, to alter it would = disaster - Strong cultural regionalism, invading armies and cultures diversified the realm - Hindu, Buddhists, Islam – strong roots in region - India is biggest power in realm, but have trouble with neighbors - Kashmir – dangerous source of friction between 2 nuclear powers II. Defining the Realm Divided along arbitrary lines drawn by England Division occurred in 1947 and many lives were lost This region includes: Pakistan (East & West), India, Bangladesh (1971), Sri Lanka, Maldives Islands Language – English is lingua franca III. Physiographic Regions of South Asia After Russia left Afghanistan Islamic revivalism entered the region Enormous range of ecologies and environment – Himalayas, desert and tropics 1) Monsoons – annual rains that are critical to life in that part of the world 1 2) Regions: A. Northern Highlands – Himalayas, Bhutan, Afghanistan B. River Lowlands – Indus Valley in Pakistan, Ganges Valley in India, Bangladesh C. Southern Plateaus – throughout much of India, a rich -

Towards Resolving Lamiales Relationships
Schäferhoff et al. BMC Evolutionary Biology 2010, 10:352 http://www.biomedcentral.com/1471-2148/10/352 RESEARCH ARTICLE Open Access Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences Bastian Schäferhoff1*, Andreas Fleischmann2, Eberhard Fischer3, Dirk C Albach4, Thomas Borsch5, Günther Heubl2, Kai F Müller1 Abstract Background: In the large angiosperm order Lamiales, a diverse array of highly specialized life strategies such as carnivory, parasitism, epiphytism, and desiccation tolerance occur, and some lineages possess drastically accelerated DNA substitutional rates or miniaturized genomes. However, understanding the evolution of these phenomena in the order, and clarifying borders of and relationships among lamialean families, has been hindered by largely unresolved trees in the past. Results: Our analysis of the rapidly evolving trnK/matK, trnL-F and rps16 chloroplast regions enabled us to infer more precise phylogenetic hypotheses for the Lamiales. Relationships among the nine first-branching families in the Lamiales tree are now resolved with very strong support. Subsequent to Plocospermataceae, a clade consisting of Carlemanniaceae plus Oleaceae branches, followed by Tetrachondraceae and a newly inferred clade composed of Gesneriaceae plus Calceolariaceae, which is also supported by morphological characters. Plantaginaceae (incl. Gratioleae) and Scrophulariaceae are well separated in the backbone grade; Lamiaceae and Verbenaceae appear in distant clades, while the recently described Linderniaceae are confirmed to be monophyletic and in an isolated position. Conclusions: Confidence about deep nodes of the Lamiales tree is an important step towards understanding the evolutionary diversification of a major clade of flowering plants. The degree of resolution obtained here now provides a first opportunity to discuss the evolution of morphological and biochemical traits in Lamiales. -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -
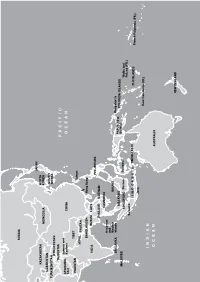
Asia and Oceania Nicole Girard, Irwin Loy, Marusca Perazzi, Jacqui Zalcberg the Country
ARCTIC OCEAN RUSSIA JAPAN KAZAKHSTAN NORTH MONGOLIA KOREA UZBEKISTAN SOUTH TURKMENISTAN KOREA KYRGYZSTAN TAJIKISTAN PACIFIC Jammu and AFGHANIS- Kashmir CHINA TAN OCEAN PAKISTAN TIBET Taiwan NEPAL BHUTAN BANGLADESH Hong Kong INDIA BURMA LAOS PHILIPPINES THAILAND VIETNAM CAMBODIA Andaman and Nicobar BRUNEI SRI LANKA Islands Bougainville MALAYSIA PAPUA NEW SOLOMON ISLANDS MALDIVES GUINEA SINGAPORE Borneo Sulawesi Wallis and Futuna (FR.) Sumatra INDONESIA TIMOR-LESTE FIJI ISLANDS French Polynesia (FR.) Java New Caledonia (FR.) INDIAN OCEAN AUSTRALIA NEW ZEALAND Asia and Oceania Nicole Girard, Irwin Loy, Marusca Perazzi, Jacqui Zalcberg the country. However, this doctrine is opposed by nationalist groups, who interpret it as an attack on ethnic Kazakh identity, language and Central culture. Language policy is part of this debate. The Asia government has a long-term strategy to gradually increase the use of Kazakh language at the expense Matthew Naumann of Russian, the other official language, particularly in public settings. While use of Kazakh is steadily entral Asia was more peaceful in 2011, increasing in the public sector, Russian is still with no repeats of the large-scale widely used by Russians, other ethnic minorities C violence that occurred in Kyrgyzstan and many urban Kazakhs. Ninety-four per cent during the previous year. Nevertheless, minor- of the population speak Russian, while only 64 ity groups in the region continue to face various per cent speak Kazakh. In September, the Chair forms of discrimination. In Kazakhstan, new of the Kazakhstan Association of Teachers at laws have been introduced restricting the rights Russian-language Schools reportedly stated in of religious minorities. Kyrgyzstan has seen a a roundtable discussion that now 56 per cent continuation of harassment of ethnic Uzbeks in of schoolchildren study in Kazakh, 33 per cent the south of the country, and pressure over land in Russian, and the rest in smaller minority owned by minority ethnic groups. -

Arctic and Boreal Plant Species Decline at Their Southern Range Limits in the Rocky Mountains
Ecology Letters, (2017) 20: 166–174 doi: 10.1111/ele.12718 LETTER Arctic and boreal plant species decline at their southern range limits in the Rocky Mountains Abstract Peter Lesica1,2* and Climate change is predicted to cause a decline in warm-margin plant populations, but this hypoth- Elizabeth E. Crone3 esis has rarely been tested. Understanding which species and habitats are most likely to be affected is critical for adaptive management and conservation. We monitored the density of 46 populations representing 28 species of arctic-alpine or boreal plants at the southern margin of their ranges in the Rocky Mountains of Montana, USA, between 1988 and 2014 and analysed population trends and relationships to phylogeny and habitat. Marginal populations declined overall during the past two decades; however, the mean trend for 18 dicot populations was À5.8% per year, but only À0.4% per year for the 28 populations of monocots and pteridophytes. Declines in the size of peripheral populations did not differ significantly among tundra, fen and forest habitats. Results of our study support predicted effects of climate change and suggest that vulnerability may depend on phylogeny or associated anatomical/physiological attributes. Keywords arctic-alpine plants, boreal plants, climate change, fens, marginal populations, peripheral popula- tions, range margins, Rocky Mountains. Ecology Letters (2017) 20: 166–174 2009; Sexton et al. 2009; Brusca et al. 2013), which suggests INTRODUCTION that in some cases climate does not determine a species’ range. Climate of the earth is changing at an unprecedented rate Nonetheless, most plant ecologists believe that climate is an (Jackson & Overpeck 2000; IPCC 2013) and is predicted to important factor determining geographic range limits. -

Albania's 'Sworn Virgins'
THE LINGUISTIC EXPRESSION OF GENDER IDENTITY: ALBANIA’S ‘SWORN VIRGINS’ CARLY DICKERSON A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS GRADUATE PROGRAM IN LINGUISTICS YORK UNIVERSITY TORONTO, ONTARIO August 2015 © Carly Dickerson, 2015 Abstract This paper studies the linguistic tools employed in the construction of masculine identities by burrneshat (‘sworn virgins’) in northern Albania: biological females who have become ‘social men’. Unlike other documented ‘third genders’ (Kulick 1999), burrneshat are not motivated by considerations of personal identity or sexual desire, but rather by the need to fulfill patriarchal roles within a traditional social code that views women as property. Burrneshat are thus seen as honourable and self-sacrificing, are accepted as men in their community, and are treated accordingly, except that they do not marry or engage in sexual relationships. Given these unique circumstances, how do the burrneshat construct and express their identity linguistically, and how do others within the community engage with this identity? Analysis of the choices of grammatical gender in the speech of burrneshat and others in their communities indicates both inter- and intra-speaker variation that is linked to gendered ideologies. ii Table of Contents Abstract ………………………………………………………………………………………….. ii Table of Contents ……………………………………………………………………………….. iii List of Tables …………………………………………………………………………..……… viii List of Figures ……………………………………………………………………………………ix Chapter One – Introduction ……………………………………………………………………... 1 Chapter Two – Albanian People and Language ………………………………………………… 6 2.0 Introduction ………………………………………………………………………………6 2.1 History of Albania ………………………………………………………………………..6 2.1.1 Geographical Location ……………………………………………………………..6 2.1.2 Illyrian Roots ……………………………………………………………………….7 2.1.3 A History of Occupations …………………………………………………………. 8 2.1.4 Northern Albania …………………………………………………………………. -

Genetic Structure and Eco-Geographical Differentiation of Lancea Tibetica in the Qinghai-Tibetan Plateau
G C A T T A C G G C A T genes Article Genetic Structure and Eco-Geographical Differentiation of Lancea tibetica in the Qinghai-Tibetan Plateau Xiaofeng Chi 1,2 , Faqi Zhang 1,2,* , Qingbo Gao 1,2, Rui Xing 1,2 and Shilong Chen 1,2,* 1 Key Laboratory of Adaptation and Evolution of Plateau Biota, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining 810001, China; [email protected] (X.C.); [email protected] (Q.G.); [email protected] (R.X.) 2 Qinghai Provincial Key Laboratory of Crop Molecular Breeding, Xining 810001, China * Correspondence: [email protected] (F.Z.); [email protected] (S.C.) Received: 14 December 2018; Accepted: 24 January 2019; Published: 29 January 2019 Abstract: The uplift of the Qinghai-Tibetan Plateau (QTP) had a profound impact on the plant speciation rate and genetic diversity. High genetic diversity ensures that species can survive and adapt in the face of geographical and environmental changes. The Tanggula Mountains, located in the central of the QTP, have unique geographical significance. The aim of this study was to investigate the effect of the Tanggula Mountains as a geographical barrier on plant genetic diversity and structure by using Lancea tibetica. A total of 456 individuals from 31 populations were analyzed using eight pairs of microsatellite makers. The total number of alleles was 55 and the number per locus ranged from 3 to 11 with an average of 6.875. The polymorphism information content (PIC) values ranged from 0.2693 to 0.7761 with an average of 0.4378 indicating that the eight microsatellite makers were efficient for distinguishing genotypes. -

Evolution in Sedges (Carex, Cyperaceae)
Evolution in sedges (Carex, Cyperaceae) A. A. REZNICEK University of Michigan Herbarium, North University Building, Ann Arbor, MI 48/09, U.S.A. Received January 2, 1990 REZNICEK,A. A. 1990. Evolution in sedges (Carex, Cyperaceae). Can. J. Bot. 68: 1409-1432. Carex is the largest and most widespread genus of Cyperaceae, but evolutionary relationships within it are poorly under- stood. Subgenus Primocarex was generally thought to be artificial and derived from diverse multispicate species. Relation- ships of rachilla-bearing species of subgenus Primocarex, however, were disputed, with some authors suggesting derivation from other genera, and others believing them to be primitive. Subgenus Indocarex, with compounded inflorescence units, was thought to be primitive, with subgenera Carex and Vignea reduced and derived. However, occurrence of rachillas is not confined to a few unispicate species, as previously thought, but is widespread. The often suggested connection between Uncinia and unispicate Carex is shown, based on rachilla morphology, to be founded on incorrect interpretation OF homology. Uncinia kingii, the alleged connecting link, is, in fact, a Carex. Unispicate Carex without close multispicate relatives probably originated from independent, ancient reductions of primitive, rachilla-bearing, multispicate Carex. The highly compounded inflorescences occumng in subgenus Vignea are hypothesized to represent a primitive state in Carex, and the more specialized inflorescences in subgenus Carex derived from inflorescences of this type. The relationships of subgenus Indocurex, with its unique perigynium-like inflorescence prophylls, remain unclear. REZNICEK,A. A. 1990. Evolution in sedges (Carex, Cyperaceae). Can. J. Bot. 68 : 1409-1432. Le Carex est le genre le plus irilportant et le plus rCpandu des Cyperaceae, mais les affinites Cvolutives a I'intCrieur de ce genre sont ma1 connues. -

Differential Response of Alpine Steppe and Alpine Meadow to Climate
Agricultural and Forest Meteorology 223 (2016) 233–240 Contents lists available at ScienceDirect Agricultural and Forest Meteorology j ournal homepage: www.elsevier.com/locate/agrformet Differential response of alpine steppe and alpine meadow to climate warming in the central Qinghai–Tibetan Plateau a,b a,b,∗ c d Hasbagan Ganjurjav , Qingzhu Gao , Elise S. Gornish , Mark W. Schwartz , a,b a,b a,b e a,b a,b Yan Liang , Xujuan Cao , Weina Zhang , Yong Zhang , Wenhan Li , Yunfan Wan , a,b f f a,b Yue Li , Luobu Danjiu , Hongbao Guo , Erda Lin a Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences, Beijing 100081, China b Key Laboratory for Agro-Environment & Climate Change, Ministry of Agriculture, Beijing 100081, China c Department of Plant Sciences, University of California, Davis 95616, USA d Institute of the Environment, University of California, Davis 95616, USA e State Key Laboratory of Water Environment Simulation, School of Environment, Beijing Normal University, Beijing 100875, China f Nagqu Agriculture and Animal Husbandry Bureau, Tibet Autonomous Region, Nagqu 852100, China a r t i c l e i n f o a b s t r a c t Article history: Recently, the Qinghai–Tibetan Plateau has experienced significant warming. Climate warming is expected Received 9 November 2015 to have profound effects on plant community productivity and composition, which can drive ecosystem Received in revised form 6 March 2016 structure and function. To explore effects of warming on plant community productivity and compo- Accepted 30 March 2016 sition, we conducted a warming experiment using open top chambers (OTCs) from 2012 to 2014 in Available online 2 May 2016 alpine meadow and alpine steppe habitat on the central Qinghai–Tibetan Plateau. -

Medicinal Plant Resources in Skuru Watershed of Karakoram Wildlife Sanctuary and Their Uses in Traditional Medicines System of Ladakh, India
International Journal of Complementary & Alternative Medicine Research Artilce Open Access Medicinal plant resources in Skuru watershed of Karakoram wildlife sanctuary and their uses in traditional medicines system of Ladakh, India Abstract Volume 11 Issue 5 - 2018 Background: The objectives of the present study were to document the medicinal Stanzin Namtak, Ramesh C Sharma plant resources of Skuru watershed in Karakoram Wildlife Sanctuary and their uses Department of Environmental Sciences, H.N.B. Garhwal in traditional medicines system (Amchi) of Ladakh. Amchi system of medicines is University (A Central University), India a complementary medicines system in Ladakh. The medicinal plants were collected in the summer season of 2015 and 2016. These medicinal plants were identified at Correspondence: Stanzin Namtak, Department of H.N.B. Garhwal University Herbarium and from some published literature. During Environmental Sciences, H.N.B. Garhwal University (A Central these surveys, 73 plant species belonging to 31 families were recorded. It was also University), Srinagar-Garhwal, 246174, Uttarakhand, India, found that maximum plant species were being in use for ailments related to digestive Email [email protected] system, followed by musculoskeletal, respiratory system, skin, cardiovascular system and blood, and nervous system. Among the plant parts used, leaves were in maximum Received: June 10, 2018 | Published: October 15, 2018 use for herbal medicines preparations followed by whole plants, flowers, shoot, roots, stem, seeds, fruits, bulbs, bark, rhizomes and tuber. Keywords: amchi, leh ladakh, medicinal plants, traditional knowledge Introduction of flora of the area.7,8 The vegetation of Ladakh is in the range of 2,700m to 6,000m a.s.l.