Lecture 9: Acetals Objectives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structures of Monosaccharides Hemiacetals
Structures of Monosaccharides Hemiacetals • Although, the open chain structures of monosaccharides are consistent with the chemistry of carbohydrates, in reality they are oversimplifications of the true structure of carbohydrates. • It is common knowledge that aldehydes react with alcohols to form hemiacetals. In cases where a molecule is a hydroxyaldehyde such as 4- hydroxybutanal or 5-hydroxypentanal, cyclic hemiacetals result. 9:47 AM 1 Structures of Monosaccharides Hemiacetals • Aldoses often contain an aldehyde group and several hydroxyl groups as part of the same molecule; they have a greater tendency of forming cyclic hemiacetals. In fact, in aqueous solution carbohydrates exist almost exclusively in the ring-closed form At equilibrium, the linear aldehyde or ketone structure represents less than 1% of the sugar present. • Five and six-membered rings are thermodynamically more stable than their corresponding four and seven membered rings, since they are less strained. • Five- (furanoses) and six-membered cyclic hemiacetals (pyranoses) are often more stable than their open-chain forms. In particular the six-membered rings which can adopt a chair conformation are 9:47 AM 2 essentially free from all types of strains. Structures of Monosaccharides Evidence for Existence of Monosacharides as Hemiacetals What physical, chemical and spectroscopic evidence support the existence of monosaccharide sugars as cyclic hemi-acetals. (a) Two anomers of glucose capable of existing independently with different physical (melting points and specific optical rotation) and chemical properties can be obtained by recrystallization. (b) the 1H-NMR and IR-spectra of solutions of pure sugars show the presence of mixtures (anomeric hemiacetals) and absence of an aldehydic peak is a sufficient indicator that the sugars exist in some other form other than the open-chain form. -

Acetal (POM) Chemical Compatibility Chart From
ver 31-Mar-2020 Acetal (POM) Chemical Compatibility Chart Chemical Chemical Acetaldehyde A Ammonium Acetate C Acetamide A Ammonium Bifluoride D Acetate Solvents A Ammonium Carbonate D Acetic Acid D Ammonium Caseinate D Acetic Acid, 20% C Ammonium Chloride, 10% B Acetic Acid, 80% D Ammonium Hydroxide D Acetic Acid, Glacial D Ammonium Nitrate, 10% A Acetic Anhydride D Ammonium Oxalate B Acetone A Ammonium Persulfate D Acetyl Chloride, dry D Ammonium Phosphate, Dibasic B Acetylene A Ammonium Phosphate, Monobasic B Alcohols: Amyl A Ammonium Phosphate, Tribasic B Alcohols: Benzyl A Ammonium Sulfate B Alcohols: Butyl A Ammonium Sulfite D Alcohols: Diacetone A Ammonium Thiosulfate B Alcohols: Ethyl A Amyl Acetate B Alcohols: Hexyl A Amyl Alcohol A Alcohols: Isobutyl A Amyl Chloride A Alcohols: Isopropyl A Aniline A Alcohols: Methyl A Aniline Oil D Alcohols: Octyl A Anise Oil D Alcohols: Propyl (1-Propanol) A Antifreeze D Aluminum chloride, 20% C Aqua Regia (80% HCl, 20% HNO3) D Aluminum Fluoride C Aromatic Hydrocarbons A Aluminum Hydroxide A Arsenic Acid D Aluminum Nitrate B Asphalt B Aluminum Potassium Sulfate, 10% C Barium Carbonate A Aluminum Potassium Sulfate, 100% C Barium Chloride A Aluminum Sulfate, 10% B Barium Cyanide B Alums C Barium Hydroxide D Amines D Barium Nitrate B Ammonia, 10% (Ammonium Hydroxide) C Barium Sulfate B Ammonia, 10% D Barium Sulfide A Ammonia, anhydrous D Bay Oil D Ammonia, liquid D Beer A Ammonia Nitrate C Beet Sugar Liquids B Key to General Chemical Resistance – All data is based on ambient or room temperature conditions, about 64°F (18°C) to 73°F (23°C) A = Excellent C = Fair - Moderate Effect, not recommended B= Good - Minor Effect, slight corrosion or discoloration D = Severe Effect, not recommended for ANY use It is the sole responsibility of the system designer and user to select products suitable for their specific application requirements and to ensure proper installation, operation, and maintenance of these products. -
![INDOLES from 2-METHYLNITROBENZENES by CONDENSATION with FORMAMIDE ACETALS FOLLOWED by REDUCTION: 4-BENZYLOXYINDOLE [1H-Indole, 4-(Phenylmethoxy)-]](https://docslib.b-cdn.net/cover/2671/indoles-from-2-methylnitrobenzenes-by-condensation-with-formamide-acetals-followed-by-reduction-4-benzyloxyindole-1h-indole-4-phenylmethoxy-222671.webp)
INDOLES from 2-METHYLNITROBENZENES by CONDENSATION with FORMAMIDE ACETALS FOLLOWED by REDUCTION: 4-BENZYLOXYINDOLE [1H-Indole, 4-(Phenylmethoxy)-]
DOI:10.15227/orgsyn.063.0214 Organic Syntheses, Coll. Vol. 7, p.34 (1990); Vol. 63, p.214 (1985). INDOLES FROM 2-METHYLNITROBENZENES BY CONDENSATION WITH FORMAMIDE ACETALS FOLLOWED BY REDUCTION: 4-BENZYLOXYINDOLE [1H-Indole, 4-(phenylmethoxy)-] Submitted by Andrew D. Batcho1 and Willy Leimgruber2. Checked by David J. Wustrow and Andrew S. Kende. 1. Procedure A. 6-Benzyloxy-2-nitrotoluene. A stirred mixture of 124.7 g (0.81 mol) of 2-methyl-3-nitrophenol (Note 1), 113.2 g (0.90 mol) of benzyl chloride, 112.2 g (0.81 mol) of anhydrous potassium carbonate, and 800 mL of dimethylformamide (DMF) is heated at 90°C for 3 hr. Most of the DMF is removed on a rotary evaporator (20 mm) and the oily residue is poured into 400 mL of 1 N sodium hydroxide and extracted with ether (3 × 800 mL). The combined extracts are dried (Na2SO4), filtered, and evaporated to give 203.5 g of yellowish solid. Recrystallization from 1 L of methanol cooled to 0°C affords 177.6 (90%) of 6-benzyloxy-2-nitrotoluene as pale-yellow crystals, mp 61–63°C3 (Note 2). B. (E)-6-Benzyloxy-2-nitro-β-pyrrolidinostyrene. To a solution of 175.4 g (0.72 mol) of 6- benzyloxy-2-nitrotoluene in 400 mL of DMF are added 102.5 g (0.84 mol) of N,N-dimethylformamide dimethyl acetal (Note 3) and 59.8 g (0.84 mol) of pyrrolidine. The solution is heated at reflux (110°C) for 3 hr (Note 4) under nitrogen and allowed to cool to room temperature. -

Handout: Organic Chemistry Reactions
Handout: Organic Chemistry Reactions Reactions Organized by Compound Families Alkanes 1. Combustion 2. Halogenation Alkenes and Alkynes 1. Additions: hydrogenation, halogenation, hydrohalogenation, hydration 2. Polymerization Aromatic Compounds Substitutions: nitration, halogenation, sulfonation Alcohols 1. Elimination: dehydration 2. Oxidations Thiols Oxidation Amines Acid-Base reactions Aldehydes and Ketones 1. Addition: Acetal/hemiacetal formation by alcohol addition (Reverse rxn: Acetal hydrolysis with acid) 2. Oxidation and Reduction for aldehydes (*Ketones go through reduction only) Carboxylic Acids 1. Substitutions: esterification, amidation (Reverse rxns: ester hydrolysis with acid or base, amide hydrolysis with acid or base) 2. Acid-base reactions Phosphoric acid and Phosphates Phosphoric acid: Esterification with alcohol Phosphates: Phosphorylation H. Kim for Chem 30B 1 Reactions Organized by Reaction Type Family Reaction Example 1. ADDITION Alkenes and Hydrogenation H H Pd catalyst H H C C + H2 H C C H alkynes H H H H H H Pd catalyst H C C H + 2 H2 H C C H H H Halogenation H H Cl Cl C C + Cl2 (or Br2) H C C H H H H H H Cl Hydro- H H Markonikov’s Rule halogenation C C + HCl (or HBr) H C C H applies: H adds to C H CH3 H CH3 with more Hs already. H OH Hydration H H H+ catalyst C C + H2O H C C H Markonikov’s Rule H CH3 H CH3 applies. Aldehydes Addition of O OH H+ catalyst H3C C H(R) + CH3CH2OH and ketones alcohol to get H3C C H(R) + CH3CH2OH hemiacetals or O CH2CH3 acetals hemiacetal O CH2CH3 H+ catalyst H3C C H(R) + H2O O CH2CH3 acetal 2. -

Aldehydes Can React with Alcohols to Form Hemiacetals
340 14 . Nucleophilic substitution at C=O with loss of carbonyl oxygen You have, in fact, already met some reactions in which the carbonyl oxygen atom can be lost, but you probably didn’t notice at the time. The equilibrium between an aldehyde or ketone and its hydrate (p. 000) is one such reaction. O HO OH H2O + R1 R2 R1 R2 When the hydrate reverts to starting materials, either of its two oxygen atoms must leave: one OPh came from the water and one from the carbonyl group, so 50% of the time the oxygen atom that belonged to the carbonyl group will be lost. Usually, this is of no consequence, but it can be useful. O For example, in 1968 some chemists studying the reactions that take place inside mass spectrometers needed to label the carbonyl oxygen atom of this ketone with the isotope 18 O. 16 18 By stirring the ‘normal’ O compound with a large excess of isotopically labelled water, H 2 O, for a few hours in the presence of a drop of acid they were able to make the required labelled com- í In Chapter 13 we saw this way of pound. Without the acid catalyst, the exchange is very slow. Acid catalysis speeds the reaction up by making a reaction go faster by raising making the carbonyl group more electrophilic so that equilibrium is reached more quickly. The the energy of the starting material. We 18 also saw that the position of an equilibrium is controlled by mass action— O is in large excess. -

Educational Research Applications Abebe M, Et Al
Educational Research Applications Abebe M, et al. Educ Res Appl 5: 175. Review Article DOI: 10.29011/2575-7032.100175 Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19 Moges Abebe1*, Lashan Eloise Knowles1, Bisrat Hailemeskel2 1Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC, USA 2Department of Clinical & Administrative Pharmacy Sciences, College of Pharmacy, Howard University, NW Washington, DC, USA *Corresponding author: Moges Abebe, Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC 27610, NC, USA Citation: Abebe M, Knowles LE, Hailemeskel B (2020) Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19. Educ Res Appl 5: 175. DOI: 10.29011/2575-7032.100175 Received Date: 26 May 2020; Accepted Date: 01 June, 2020; Published Date: 06 June, 2020 Abstract End of semester organic chemistry course projects are valuable learning assessment tools while giving students a creative opportunity and sparking interest for further research investigations. The purpose of this year’s project was to teach students how to synthesize a molecule that potentially mimics an existing drug that works against the COVID-19. The available drugs chosen for the project are those that are proposed to work either by prohibiting the easy entry of the virus into respiratory tissues or those who deprive the virus’s ability to reproduce once they enter the cell. An investigative search in historical literature and the current conditions of the virus enabled students to create a unique and innovative product that requires a cumulative learned knowledge. History has shown that when a new virus becomes pandemic it takes time for researchers to create a drug, test the results, and gets approved by the Food and Drug Administration (FDA) for public availability. -

Chapter 3. the Concept of Protecting Functional Groups
Chapter 3. The Concept of Protecting Functional Groups When a chemical reaction is to be carried out selectively at one reactive site in a multifunctional compound, other reactive sites must be temporarily blocked. A protecting group must fulfill a number of requirements: • The protecting group reagent must react selectively (kinetic chemoselectivity) in good yield to give a protected substrate that is stable to the projected reactions. • The protecting group must be selectively removed in good yield by readily available reagents. • The protecting group should not have additional functionality that might provide additional sites of reaction. 3.1 Protecting of NH groups Primary and secondary amines are prone to oxidation, and N-H bonds undergo metallation on exposure to organolithium and Grignard reagents. Moreover, the amino group possesses a lone pair electrons, which can be protonated or reacted with electrophiles. To render the lone pair electrons less reactive, the amine can be converted into an amide via acylation. N-Benzylamine Useful for exposure to organometallic reagents or metal hydrides Hydrogenolysis Benzylamines are not cleaved by Lewis acid Pearlman’s catalyst Amides Basicity of nitrogen is reduced, making them less susceptible to attack by electrophilic reagent The group is stable to pH 1-14, nucleophiles, organometallics (except organolithium reagents), catalytic hydrogenation, and oxidation. Cleaved by strong acid (6N HCl, HBr) or diisobutylaluminum hydride Carbamates Behave like a amides, hence no longer act as nucleophile Stable to oxidizing agents and aqueous bases but may react with reducing agents. Iodotrimethylsilane is often the reagent for removal of this protecting group Stable to both aqueous acid and base Benzoyloxycarbonyl group for peptide synthesis t-butoxycarbonyl group(Boc) is inert to hydrogenolysis and resistant to bases and nucleophilic reagent. -

Structures of Monosaccharides Hemiacetals
Disaccharides 10:51 AM 1 Disaccharides Definition • Disaccharides are carbohydrates consisting of two monosaccharide units linked via a glycosidic bond. Non-reducing disaccharide (1,1'-Glycosidic linkage) OH HO OH O HO O OH O OH OH HO OH HO O O HO OH + HO OH Glycosidic bond OH OH HO OH HO OH 6' 6 O O Reducing end 5' 1' 4 5 HO 4' O OH 3' 2' 3 2 1 HO OH HO OH Glycone Aglycone Reducing disaccharide (1,4'-Glycosidic linkage) • These disaccharides may be reducing or non-reducing sugars depending on the regiochemistry of the glycosidic 10:51 AM linkage between the two monosaccharides. 2 Nomenclature of Disaccharides • Since disaccharides are glycosides with two monosaccharide units linked through a glycosidic bond, their nomenclature requires the formulation of priority rules to identify which of the two monosaccharides of a disaccharide provides the parent name of the disaccharide and which one will be considered the substituent. • The nomenclature of disaccharides is based on the following considerations: i. Disaccharides with a free hemiacetal group (Reducing disaccharide) ii. Disaccharides without a free hemiacetal group (Non- Reducing Disaccharide) 10:51 AM 3 Nomenclature of Reducing Disaccharides • A disaccharide in which one glycosyl unit appears to have replaced the hydrogen atom of a hydroxyl group of the other is named as a glycosylglycose. The locants of the glycosidic linkage and the anomeric descriptor(s) must be given in the full name. • The parent sugar residue in such a reducing disaccharide is chosen on the basis of the following criteria: • The parent sugar residue is the one that includes the functional group most preferred by general principles of organic nomenclature. -

Hydration of Ketones and Aldehydes
Hydration of Ketones and Aldehydes H H H O O H H H H HO OH H2O O O HO OH O a hydrated ketone H H O HO OH H2O Keq = 2000 uncrowded H H H H Aldehydes exist as partial hydrates in aqueous solution: O HO OH H2O Keq = 1 more crowded Me H Me H O H O HO OH Ketones generally do not favor hydration: 2 Keq = 0.002 very crowded Me Me Me Me Nucleophilic Addition of "O–" to Carbonyl Groups Na O O OH H+ (workup) O HO Na + H2O Me Me Me Me Me Me OH OH Me Me A hydrated ketone Starting material! pKa of H2O: approx. 16 pKa of alkoxide: 16-20 Na O O OH H+ (workup) O MeO Na + MeOH Me Me Me Me Me Me OMe OMe Me Me A hemiacetal Starting material! (usually unstable) pKa of MeOH: approx. 15 pKa of alkoxide: 16-20 Hydration of Ketones and Aldehydes 18 O 18 O H2 O + Me Me H cat. Me Me H H H 18O 18O H H H Me Me H H Me 18 18 O O H O OH O H H Me 18O Me Me H H H18O OH H Me Me 18 H O a hydrated ketone Me Me H 18 H O OH2 18 O 18O Me Me Me Me Addition of Alcohols to Carbonyl Groups: Acetal Formation O ROH, H+ RO OR Me Me Me Me An acetal MeOH H+ Me Me H H Me O O MeO OH H+ Me MeOHMe Me MeO OH MeOH Me Me a hemiacetal MeO OMe H Me Me Me MeO OH2 Me Me MeO OMe O Me Me Me An acetal Me MeOH H2O Acetals as Carbonyl Protecting Groups O OH MeMgCl Me Me Me Me Me MeO OMe MeMgCl No reaction Me Me O Br O Li–Bu O P(Ph)3 Br (Ph)3P X (Ph)3P Me Me Me Butyllithium will react with the ketone, and the reagent will react with itself! O MeO OMe MeOH, H+ MeO OMe (Ph) P Br Br 3 Me Me Me A stable reagent O O MeO OMe + H2O, H Me Me Cyclic Hemiacetals MeOH H+ Me H Me MeOH Me H MeO OH O O MeO OH Me -

Chapter 16 ! Bonds with Hidden Leaving Groups (Sections 16.1-16.6 Excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals
O Chemistry 2600 Chapter 16 ! Bonds With Hidden Leaving Groups (sections 16.1-16.6 excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals • In Chapters 7 and 15 we saw how carbonyl compounds undergo addition reactions with nucleophiles. • We also saw how some of these reactions are reversible. • For example, recall the formation of a hydrate when an aldehyde or ketone reacts with water under acidic condition: 2 O Formation and Reactivity of Acetals • This reaction is reversible and after the loss of a leaving group, the carbonyl group is reformed: • Note that the oxygen that is lost could come from the initial carbonyl oxygen atom. • We say these molecules have a ‘hidden leaving group’. • This chapter explores the chemistry of the removal or replacement of these hidden leaving groups. 3 O Formation and Reactivity of Acetals • When an aldehyde/ketone reacts with an alcohol in the presence of acid, a hemiacetal is formed. • The only difference between hemiacetal formation and hydrate formation is the nucleophile (water vs alcohol). • Hemiacetals have a hidden leaving group and in the presence of alcohol and acid, react quickly to form an acetal. • Hemiacetal (tetrahedral carbon attached to –OH and –OR) • Acetal (tetrahedral carbon attached to two –OR groups) 4 O Formation and Reactivity of Acetals • Acetal formation: 5 O Formation and Reactivity of Acetals • Acetal formation is an equilibrium process, all the steps in the sequence can run in both directions. • When an acetal is converted to a carbonyl compound, the reaction is called hydrolysis because water is being used to break (lyse) the acetal. -

20H-Carbohydrates.Pdf
Carbohydrates Carbohydrates are compounds that have the general formula CnH2nOn Because CnH2nOn can also be written Cn(H2O)n, they appear to be “hydrates of carbon” Carbohydrates are also called “sugars” or “saccharides” Carbohydrates can be either aldoses (ald is for aldehyde and ose means a carbohydrate) or ketoses (ket is for ketone) OH OH O OH CH2OH CH2OH OHC HOH2C OH OH OH OH An Aldose A Ketose (D-Glucose) (D-Fructose) Carbohydrates Due to the multiple chiral centers along a linear carbon chain for carbohydrates, Emil Fischer developed the “Fischer Projection” in order to represent these compounds Remember how to draw a Fischer projection: 1) View the linear carbon chain along the vertical axis (always place the more oxidized carbon [aldehyde in an aldose] towards the top) 2) The horizontal lines are coming out of the page toward the viewer 3) Will need to change the viewpoint for each carbon so the horizontal substituents are always pointing towards the viewer CHO OH OH H OH HO H CH2OH = OHC H OH OH OH H OH CH2OH Emil Fischer (1852-1919) Carbohydrates The aldoses are thus all related by having an aldehyde group at one end, a primary alcohol group at the other end, and the two ends connected by a series of H-C-OH groups CHO CHO CHO CHO CHO H OH H OH H OH H OH HO H CH2OH H OH H OH H OH HO H CH2OH H OH H OH HO H CH2OH H OH HO H CH2OH CH2OH Aldotriose Aldotetrose Aldopentose Aldohexose Aldohexose D-glyceraldehyde D-erythose D-ribose D-allose L-allose The D-aldoses are named according to glyceraldehyde, the D refers to the configurational -
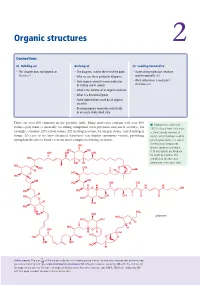
Organic Structures 2
Organic structures 2 Connections Building on Arriving at Looking forward to • This chapter does not depend on • The diagrams used in the rest of the book • Ascertaining molecular structure Chapter 1 • Why we use these particular diagrams spectroscopically ch3 • How organic chemists name molecules • What determines a molecule’s in writing and in speech structure ch4 • What is the skeleton of an organic molecule • What is a functional group • Some abbreviations used by all organic chemists • Drawing organic molecules realistically in an easily understood style There are over 100 elements in the periodic table. Many molecules contain well over 100 ■ Palytoxin was isolated in atoms—palytoxin (a naturally occurring compound with potential anticancer activity), for 1971 in Hawaii from Limu make example, contains 129 carbon atoms, 221 hydrogen atoms, 54 oxygen atoms, and 3 nitrogen o Hane (‘deadly seaweed of atoms. It’s easy to see how chemical structures can display enormous variety, providing Hana’), which had been used to enough molecules to build even the most complicated living creatures. poison spear points. It is one of the most toxic compounds OH known, requiring only about HO OH 0.15 micrograms per kilogram OH OH OH for death by injection. The HO complicated structure was O OH H determined a few years later. HO OH OH O H H O O HO HO H OH OH HO OH OH H H H H O N N OH HO HO H HO OH OH O O O HO OH H OH HO OH NH2 HO OH HO palytoxin HO OH O H HO H OH O H H H HO HO OH O O OH OH HO H Online support.