Atomic Structure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chlorine Stable Isotopes in Sedimentary Systems: Does Size Matter?
Chlorine stable isotopes in sedimentary systems: does size matter? Max Coleman Jet Propulsion Laboratory, Caltech, Pasadena, California, USA ABSTRACT: Stable isotope abundances vary because of size (atomic mass) differences. The chlorine stable isotope system was one of the first described theoretically, but had a slow, disappointment-strewn develop- ment, relative to other elements. Method improvement gave only small, but significant, differences (-1 %.) in compositions of geological materials. Eventually, brines and groundwater chlorides gave larger differences (-5%0). Physical processes like diffusion and adsorption, probably are the main controls of groundwater com- positions. In contrast, the processes producing brine compositions are still enigmatic and need further work for full understanding. Recent work on anthropogenic groundwater contaminants (chlorinated aliphatic hy- drocarbons and perchlorate) shows variations resulting from manufacturing processes; implying possibilities of tracing sources. However, they are subject to microbial degradation, producing much larger fractionations (115%0),but therefore offering better possibility of monitoring natural attenuation. So atomic size is important to give isotopic fractionation and the size of fractionation matters, allowing observation of otherwise unde- tectable processes. lecular and atomic weights by weighing the gases and relating them to the weight of hydrogen. All 1 INTRODUCTION atomic weights were integer multiples the weight of This paper aims to give a short and selective -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

Unit 1Moles and Equations
Cambridge University Press 978-1-107-61665-3 – Cambridge International AS and A Level Chemistry Judith Potter Peter Cann Excerpt More information Moles and equations Unit 1 Learning outcomes You should be able to: defi ne and use the terms empirical and molecular formulae and use experimental data defi ne and use relative masses based on the to fi nd them, 12C scale write and construct equations and use them in defi ne and use the mole and Avogadro’s performing mole calculations constant perform mole calculations involving solutions analyse simple mass spectra and use the data in and gases, relative atomic mass calculations This unit gathers together the basic chemistry relative isotopic mass = calculations. You may need to refer to it as you revise mass of one atom of the isotope other topics in later units. (1/12) of the mass of one atom of 12C Relative masses are a ratio of two masses and have 1.01 Relative masses no units. An atom is the smallest part of an element that can exist and still retain the identity of the element. A 1.02 The mole and the molecule is a group of atoms bonded together and the smallest portion of an element or compound that can Avogadro constant exist alone. A simple ion is an atom which has lost or To give an easy measurement of mass, a number of gained one or more electrons. Atoms, molecules and atoms is chosen so that their combined mass is equal to ions are so small that their masses are far too small the relative atomic mass in grams. -

Chromium Compounds Hazard Summary
Chromium Compounds Hazard Summary Chromium occurs in the environment primarily in two valence states, trivalent chromium (Cr III) and hexavalent chromium (Cr VI). Exposure may occur from natural or industrial sources of chromium. Chromium III is much less toxic than chromium (VI). The respiratory tract is also the major target organ for chromium (III) toxicity, similar to chromium (VI). Chromium (III) is an essential element in humans. The body can detoxify some amount of chromium (VI) to chromium (III). The respiratory tract is the major target organ for chromium (VI) toxicity, for acute (short-term) and chronic (long-term) inhalation exposures. Shortness of breath, coughing, and wheezing were reported from a case of acute exposure to chromium (VI), while perforations and ulcerations of the septum, bronchitis, decreased pulmonary function, pneumonia, and other respiratory effects have been noted from chronic exposure. Human studies have clearly established that inhaled chromium (VI) is a human carcinogen, resulting in an increased risk of lung cancer. Animal studies have shown chromium (VI) to cause lung tumors via inhalation exposure. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (7), which contains information on inhalation chronic toxicity and the RfC and oral chronic toxicity and the RfD, and the carcinogenic effects of chromium including the unit cancer risk for inhalation exposure, EPA's Toxicological Review of Trivalent Chromium and Toxicological Review of Hexavalent Chromium (3), and the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Chromium. (1) Uses The metal chromium is used mainly for making steel and other alloys. -

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

Chromium Isotopes Detection in Their Ores with Minimal Errors
Open Access Annals of Advances in Chemistry Research Article Chromium Isotopes Detection in their Ores with Minimal Errors ISSN 2576-3768 Loai Aljerf1* and Nuha AlMasri2 1Department of Basic Sciences, Faculty of Dental Medicine, Damascus University, Mazzeh Highway, Almazzeh, Damascus, Syria 2Department of Chemistry, Faculty of Medicine, Syrian Private University, Damascus, Syria *Address for Correspondence: Loai Aljerf, De- Abstract partment of Basic Sciences, Faculty of Dental Medicine, Damascus University, Mazzeh High- The industrial production and use of chromium have grown considerably during the past way, Almazzeh, Damascus, Syria, Tel: +963-93 fi ve decades. Abundances of the chromium isotopes in terrestrial samples are identical to 34 46 993; Email: [email protected]; 0.01%. Among the dominant species of chromium, the trivalent form widely occurs in nature in [email protected] chromite ores which is extremely immobilized especially in water bodies. Samples were mixtures Submitted: 21 July 2018 of separated chromium isotopes and the calibration was made with the same species as those Approved: 03 September 2018 used in the measurements. The method had simplifi ed the conversion of the ores to chromyl Published: 04 September 2018 fl uoride since the element could be readily separated as lead chromate from the leaching of Copyright: 2018 Aljerf L, et al. This is an open chromite-sodium peroxide fusions. Isotope assay of chromyl fl uoride under certain conditions access article distributed under the Creative was measured and the measurements of chromium isotopic anomalies ratios and isotope Commons Attribution License, which permits abundance of the chromite ores have been assessed. These provided suffi cient quantitative mass unrestricted use, distribution, and reproduction spectrometric data, which were analyzed to calculate the abundance and the mean atomic mass in any medium, provided the original work is of the questioned isotopes. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -

Determination of Picogram Quantities of Vanadium in Calcite And
+ + Anal. Chem. 1996, 68, 371-377 Determination of Picogram Quantities of Vanadium in Calcite and Seawater by Isotope Dilution Inductively Coupled Plasma Mass Spectrometry with Electrothermal Vaporization David W. Hastings* and Steven R. Emerson School of Oceanography, WB-10, University of Washington, Seattle, Washington 98195 Bruce K. Nelson Department of Geological Sciences, AJ-20, University of Washington, Seattle, Washington 98195 We developed a method to measure picogram quantities Recent interest in the geochemistry of vanadium has arisen of vanadium in calcite and seawater by isotope dilution from its unique redox behavior 9,10 and the observation of enriched (ID) inductively coupled plasma mass spectrometry using concentrations in anoxic sediments, black shale, and crude oil.11,12 electrothermal vaporization (ETV) to introduce the sample Because V deposition is redox sensitive, its concentration in into the plasma. A 50V isotope spike enriched to 44 atom seawater is most likely sensitive to the oxidation state of sedi- % was equilibrated with samples, followed by chemical ments. The calcitic shells of foraminifera accurately record purification by cation exchange chromatography. Samples seawater concentrations of V,13 so the V content of the fossil shells were introduced into the ETV unit with a Pd modifier and of these microscopic oceanic organisms potentially can serve as heated to 1000 °C. This quantitatively eliminates the an indicator of changes in past ocean anoxia.14 ClO+ isobaric interference with V at m/z 51 for solutions A typical 5 mg hand-picked sample of these microscopic shells up to 0.5 N HCl. The procedural blank was 0.27 pg of yields only 50-200 pg of vanadium. -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -
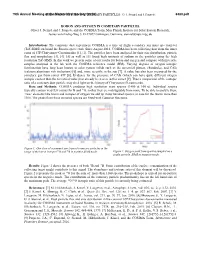
Boron and Oxygen in Cometary Particles: O. J
79th Annual Meeting ofBORON the Meteoritical AND OXYGEN Society IN COMETARY (2016) PARTICLES: O. J. Stenzel and J. Paquette 6380.pdf BORON AND OXYGEN IN COMETARY PARTICLES. Oliver J. Stenzel and J. Paquette and the COSIMA Team, Max Planck Institute for Solar System Research, Justus-von-Liebig-Weg 3, D-37077 Göttingen, Germany, [email protected]. Introduction: The cometary dust experiment COSIMA is a time of flight secondary ion mass spectrometer (ToF-SIMS) on board the Rosetta space craft. Since August 2014, COSIMA has been collecting dust from the inner coma of 67P/Churyumov-Gerasimenko [1], [2]. The particles have been analyzed for their size distribution, particle flux and morphology [3], [4]. [5] as well as [1] found high amounts of sodium in the particles using the high resolution ToF-SIMS. In this work we present some of our results for boron and oxygen and compare with meteorite samples analyzed in the lab with the COSIMA reference model (RM). Varying degrees of oxygen isotopic fractionation have long been known in solar system solids such as the terrestrial planets, chondrules, and CAIs (calcium-aluminum-rich inclusions) [6] and, more recently, in the sun [7]. A value has also been measured for the cometary gas from comet 67P [8]. Evidence for the presence of CAIs (which can have quite different oxygen isotopic content than the terrestrial value) has already been seen in this comet [9]. Thus a comparison of the isotopic ratio of a cometary dust particle may shed light on the history of Churyumov-Gerasimenko. Data and Methods: COSIMA produces high resolution mass spectra (1400 at 100 u). -
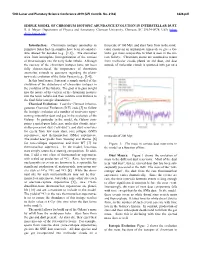
Simple Model of Chromium Isotopic Abundance Evolution in Interstellar Dust
50th Lunar and Planetary Science Conference 2019 (LPI Contrib. No. 2132) 3226.pdf SIMPLE MODEL OF CHROMIUM ISOTOPIC ABUNDANCE EVOLUTION IN INTERSTELLAR DUST. B. S. Meyer,. Department of Physics and Astronomy, Clemson University, Clemson, SC 29634-0978, USA (mbra- [email protected]). Introduction: Chromium isotopic anomalies in timescale of 100 Myr, and stars form from in the mole- primitive Solar System samples have been of consider- cular clouds on an appropriate timescale to give a Ga- able interest for decades (e.g., [1,2]). The anomalies lactic gas mass comparable to what is seen in the cur- arise from incomplete homogenization of the carriers rent Galaxy. Chromium atoms are assumed to return of these isotopes into the early Solar nebula. Although from molecular clouds plated on old dust, and dust the carriers of the chromium isotopes have not been outside of molecular clouds is sputtered into gas on a fully characterized, the importance of chromium anomalies extends to questions regarding the plane- tary-scale evolution of the Solar System (e.g., [3,4]). In this brief paper, I present a simple model of the evolution of the abundances of chromium isotopes in the evolution of the Galaxy. The goal is to gain insight into the nature of the carriers of the chromium isotopes into the Solar nebula and their relative contributions to the final Solar isotopic abundances. Chemical Evolution: I use the Clemson Inhomo- geneous Chemical Evolution (ICE) code [5] to follow the isotopic evolution of a number of reservoirs repre- senting interstellar dust and gas in the evolution of the Galaxy. -

Investigation of Nitrogen Cycling Using Stable Nitrogen and Oxygen Isotopes in Narragansett Bay, Ri
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2014 INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI Courtney Elizabeth Schmidt University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Schmidt, Courtney Elizabeth, "INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI" (2014). Open Access Dissertations. Paper 209. https://digitalcommons.uri.edu/oa_diss/209 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI BY COURTNEY ELIZABETH SCHMIDT A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN OCEANOGRAPHY UNIVERSITY OF RHODE ISLAND 2014 DOCTOR OF PHILOSOPHY DISSERTATION OF COURTNEY ELIZABETH SCHMIDT APPROVED: Thesis Committee: Major Professor Rebecca Robinson Candace Oviatt Arthur Gold Nassar H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2014 ABSTRACT Estuaries regulate nitrogen (N) fluxes transported from land to the open ocean through uptake and denitrification. In Narragansett Bay, anthropogenic N loading has increased over the last century with evidence for eutrophication in some regions of Narragansett Bay. Increased concerns over eutrophication prompted upgrades at wastewater treatment facilities (WWTFs) to decrease the amount of nitrogen discharged. The upgrade to tertiary treatment – where bioavailable nitrogen is reduced and removed through denitrification – has occurred at multiple facilities throughout Narragansett Bay’s watershed.