Catalogue About 49 Indium Base Presentation and Precious Metal Recycling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Densifying Metal Hydrides with High Temperature and Pressure
3,784,682 United States Patent Office Patented Jan. 8, 1974 feet the true density. That is, by this method only theo- 3,784,682 retical or near theoretical densities can be obtained by DENSIFYING METAL HYDRIDES WITH HIGH making the material quite free from porosity (p. 354). TEMPERATURE AND PRESSURE The true density remains the same. Leonard M. NiebylsM, Birmingham, Mich., assignor to Ethyl Corporation, Richmond, Va. SUMMARY OF THE INVENTION No Drawing. Continuation-in-part of abandoned applica- tion Ser. No. 392,370, Aug. 24, 1964. This application The process of this invention provides a practical Apr. 9,1968, Ser. No. 721,135 method of increasing the true density of hydrides of Int. CI. COlb 6/00, 6/06 metals of Groups II-A, II-B, III-A and III-B of the U.S. CI. 423—645 8 Claims Periodic Table. More specifically, true densities of said 10 metal hydrides may be substantially increased by subject- ing a hydride to superatmospheric pressures at or above ABSTRACT OF THE DISCLOSURE fusion temperatures. When beryllium hydride is subjected A method of increasing the density of a hydride of a to this process, a material having a density of at least metal of Groups II-A, II-B, III-A and III-B of the 0.69 g./cc. is obtained. It may or may not be crystalline. Periodic Table which comprises subjecting a hydride to 15 a pressure of from about 50,000 p.s.i. to about 900,000 DESCRIPTION OF THE PREFERRED p.s.i. at or above the fusion temperature of the hydride; EMBODIMENT i.e., between about 65° C. -

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Doped Lanthanum Manganite Thin Films for SOFC Application
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 5, ISSUE 04, APRIL 2016 ISSN 2277-8616 Spectroscopic And Electrical Properties Of Strontium - Doped Lanthanum Manganite Thin Films For SOFC B. S. Kamble, V. J. Fulari, R. K. Nimat Abstract: Strontium-doped lanthanum manganite (La1-xSrxMnO3-δ-LSM) has received much attention in recent years. Most studies have concentrated on thin film samples at low temperatures. La1-xSrxMnO3-δ (LSM) has proved to be efficient cathode material for solid oxide fuel cell (SOFC). In the present work the strontium-doped lanthanum manganite (La0.9Sr0.1MnO3-δ) were synthesized by spray pyrolysis technique in the thin film form on alumina substrate which was characterized by X-ray diffraction, scanning electron microscopy. The spectroscopic properties such as IR, FT-Raman have been studied. The temperature dependence resistivity and Dielectric constant was carried out at different frequencies. Film thickness measurement was carried out by using surface profilometer. X-ray diffraction results clearly indicated that sintered LSM at 900oC for 2 hours show the formation of single phase solid solution with pervoskite like structure. Crystalline size has been calculated by SEM microscopy. Resistivity of LSM thin film decreases as absolute temperature increases and Dielectric constant suddenly decreases at low frequency and became constant as frequency increases. The film thickness is about 200 nm and roughness of the sample is 4000Ao. Keywords: Spray Pyrolysis, Cathode, LSM, SOFC. ———————————————————— 1. Introduction: The strontium doped lanthanum manganite La1-xMxMnO3, The power generation by clean, efficient and eco-friendly is where, M=Alkaline earth metal, plays the attention as the biggest challenge for scientists and engineers. -

Reactions of Lithium Nitride with Some Unsaturated Organic Compounds. Perry S
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1963 Reactions of Lithium Nitride With Some Unsaturated Organic Compounds. Perry S. Mason Jr Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Mason, Perry S. Jr, "Reactions of Lithium Nitride With Some Unsaturated Organic Compounds." (1963). LSU Historical Dissertations and Theses. 898. https://digitalcommons.lsu.edu/gradschool_disstheses/898 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 64—5058 microfilmed exactly as received MASON, Jr., Perry S., 1938- REACTIONS OF LITHIUM NITRIDE WITH SOME UNSATURATED ORGANIC COMPOUNDS. Louisiana State University, Ph.D., 1963 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. REACTIONS OF LITHIUM NITRIDE WITH SOME UNSATURATED ORGANIC COMPOUNDS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requireiaents for the degree of Doctor of Philosophy in The Department of Chemistry by Perry S. Mason, Jr. B. S., Harding College, 1959 August, 1963 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. -

UCLA UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Tetrides and Pnictides for Fast-Ion Conductors, Phosphor-Hosts, Structural Materials and Improved Thermoelectrics Permalink https://escholarship.org/uc/item/9068g8b2 Author Hick, Sandra Marie Publication Date 2013 Supplemental Material https://escholarship.org/uc/item/9068g8b2#supplemental Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Tetrides and Pnictides for Fast-Ion Conductors, Phosphor-Hosts, Structural Materials and Improved Thermoelectrics A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Chemistry by Sandra Marie Hick 2013 ABSTRACT OF THE DISSERTATION Tetrides and Pnictides for Fast-Ion Conductors, Phosphor-Hosts, Structural Materials and Improved Thermoelectrics by Sandra Marie Hick Doctor of Philosophy in Chemistry University of California, Los Angeles, 2013 Professor Richard B. Kaner, Chair New routes to solid states materials are needed for discovery and the realization of improved reactions. By utilizing reactive approaches such as solid-state metathesis, pyrolysis, and nitride fluxes new routes to hard materials, phosphor hosts, and fast ion conductors were developed. The fast ion conductor lithium silicon nitride, Li2SiN2, was produced in a metathesis reaction between silicon chloride, SiCl4 and lithium nitride, Li3N, initiated in a conventional microwave oven. The Li2SiN2 produced had a conductivity of 2.70 x 10-3 S/cm at 500 °C. Colorless millimeter-sized crystals of Ca16Si17N34 were synthesized at high temperatures from a flux generated in situ from reaction intermediates. The ii compound was found to crystallize in the cubic space group F-43m (a = 14.8882 Å). -

CHEMISTRY 1. A. Coursed Offered
CHEMISTRY Phone : + 91-33-2414 6223 Fax : + 91-33- 2414 6223 Int. : 2457-2267/2765 2457-2147(Inorganic)/ 2104 (Organic)/ 2458(Physical)/2453 (Radiochemistry) H.O.D. : Prof. Subhash Chandra Bhattacharya E-mail : [email protected] [email protected] 1. A. Coursed Offered: Name of the Course Duration of the Course No. of Students B.Sc. 3 years 55 × 3 M.Sc. (Day) 2 years 40 × 2 M.Sc. (Evening) 3 years 35 × 2 B. Number of Faculty Members: Professor Reader/Associate Professor Assistant Professor 18 9 16 2. A. Area Research Activities: Prof. Chittaranjan Sinha Studies on the coordination chemistry, characterisation and the application potential of the transition and nontransition metal complexes. Design of azo and imine functionalised molecules of N- heterocycles (pyridine, pyrimidine, pyrazole, imidazole), sulphonamide antibiotics such as sulfamethoxazole; the structural studies, exploration of chemical, biochemical, photophysical, mesogenic activity and redox properties of the compounds are main target of our group. Metallo- organic, organometallics, supramolecular chemistry, photovoltaic activity and photochromism, antimicrobial activity and cytotoxicity of molecules are broad field of study. Theoretical computation and in-silico study of the compounds are our objective. Prof. Nitin Chattopadhyay Photochemistry; Biophysical Chemistry; Surface Chemistry; Fluorescence sensing; Polymer photophysics; Nanoparticle research; Steady-state and time-resolved fluorometric study of the photophysical and photochemical processes (ESPT, ICT, electron transfer, charge transfer and energy transfer) in homogeneous as well as microheterogeneous environments; Binding of drugs with proteins and DNA; Theoretical modeling and simulations of photophysical and/or photochemical observations; Laser induced optoacoustic spectroscopy (LIOAS). Prof. Subhas Chandra Bhattacharya 1. Photophysical and Photochemical processes in organised interfaces. -

Reduction of Rocksalt Phase in Ag-Doped Ge2sb2te5: a Potential Material for Reversible Near-Infrared Window
PHYSICAL REVIEW APPLIED 10, 054070 (2018) Reduction of Rocksalt Phase in Ag-Doped Ge2Sb2Te5: A Potential Material for Reversible Near-Infrared Window Palwinder Singh,1,2 A.P. Singh,3 Jeewan Sharma,4 Akshay Kumar,4 Monu Mishra,5 Govind Gupta,5 and Anup Thakur2,* 1 Department of Physics, Punjabi University, Patiala, Punjab 147002, India 2 Advanced Materials Research Lab, Department of Basic and Applied Sciences, Punjabi University, Patiala, Punjab 147002, India 3 Department of Physics, Dr. B. R. Ambedkar National Institute of Technology, Jalandhar 144011, India 4 Department of Nanotechnology, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Punjab 140407, India 5 Advanced Materials and Devices Division, CSIR-National Physical Laboratory, New Delhi 110012, India (Received 15 July 2018; revised manuscript received 1 November 2018; published 30 November 2018) Phase-change materials are attracting much attention in the scientific and engineering communities owing to their applications and underlying basic phenomena. Ge2Sb2Te5 is reversible-phase-change mate- rial (amorphous to crystalline and vice versa) that is used for optical data storage and phase-change random-access memory and has recently been explored for use as a reversible near-infrared (NIR) win- dow [Singh et al., Appl. Phys. Lett. 111, 261102 (2017)]. For a reversible NIR window, large transmission contrast between two phases and low phase-transition temperature are required to reduce the power con- ( ) = sumption. In the present work, phase transition in thermally deposited Ge2Sb2Te5 100−xAgx (x 0, 1, 3, 5, and 10) thin films is achieved by vacuum thermal annealing. Transmission sharply decreases with phase transition in the NIR region. -

Phase-Change Properties of Gesbte Thin Films Deposited by Plasma
Song et al. Nanoscale Research Letters (2015) 10:89 DOI 10.1186/s11671-015-0815-5 NANO EXPRESS Open Access Phase-change properties of GeSbTe thin films deposited by plasma-enchanced atomic layer depositon Sannian Song1*, Dongning Yao1, Zhitang Song1, Lina Gao2, Zhonghua Zhang1,LeLi1, Lanlan Shen1, Liangcai Wu1, Bo Liu1, Yan Cheng1 and Songlin Feng1 Abstract Phase-change access memory (PCM) appears to be the strongest candidate for next-generation high-density nonvolatile memory. The fabrication of ultrahigh-density PCM depends heavily on the thin-film growth technique for the phase-changing chalcogenide material. In this study, Ge2Sb2Te5 (GST) and GeSb8Te thin films were deposited by plasma-enhanced atomic layer deposition (ALD) method using Ge [(CH3)2 N]4,Sb[(CH3)2 N]3,Te (C4H9)2 as precursors and plasma-activated H2 gas as reducing agent of the metallorganic precursors. Compared with GST-based device, GeSb8Te-based device exhibits a faster switching speed and reduced reset voltage, which is attributed to the growth-dominated crystallization mechanism of the Sb-rich GeSb8Te films. These results show that ALD is an attractive method for preparation of phase-change materials. Keywords: Phase-change memory; Atomic layer deposition; Microstructure; Electric properties Background of the limited on-current drive capability of the cell tran- Phase-change memory (PCM) has been regarded as one sistor [6]. It has been reported that a confined cell struc- of the most promising nonvolatile memories for the next ture where the phase-change material is formed inside a generation because of the advantages of high speed, low contact via is expected to be essential for the next- power, good endurance, high scalability, and fabrication generation PCM device because it requires lower switch- compatibility with complementary metal-oxide semicon- ing power [7,8]. -

PRAJNA - Journal of Pure and Applied Sciences ISSN 0975 2595 Volume 19 December 2011 CONTENTS
PRAJNA - Journal of Pure and Applied Sciences ISSN 0975 2595 Volume 19 December 2011 CONTENTS BIOSCIENCES Altered energy transfer in Phycobilisomes of the Cyanobacterium, Spirulina Platensis under 1 - 3 the influence of Chromium (III) Ayya Raju, M. and Murthy, S. D. S. PRAJNA Volume 19, 2011 Biotransformation of 11β , 17 α -dihydroxy-4-pregnene-3, 20-dione-21-o-succinate to a 4 - 7 17-ketosteroid by Pseudomonas Putida MTCC 1259 in absence of 9α -hydroxylase inhibitors Rahul Patel and Kirti Pawar Influence of nicking in combination with various plant growth substances on seed 8 - 10 germination and seedling growth of Noni (Morinda Citrifolia L.) Karnam Jaya Chandra and Dasari Daniel Gnana Sagar Quantitative analysis of aquatic Macrophytes in certain wetlands of Kachchh District, 11 - 13 Journal of Pure and Applied Sciences Gujarat J.P. Shah, Y.B. Dabgar and B.K. Jain Screening of crude root extracts of some Indian plants for their antibacterial activity 14 - 18 Purvesh B. Bharvad, Ashish R. Nayak, Naynika K. Patel and J. S. S. Mohan ________ Short Communication Heterosis for biometric characters and seed yield in parents and hybrids of rice 19 - 20 (Oryza Sativa L.) M. Prakash and B. Sunil Kumar CHEMISTRY Adsorption behavior and thermodynamics investigation of Aniline-n- 21 - 24 (p-Methoxybenzylidene) as corrosion inhibitor for Al-Mg alloy in hydrochloric acid V.A. Panchal, A.S. Patel and N.K. Shah Grafting of Butyl Acrylate onto Sodium Salt of partially Carboxymethylated Guar Gum 25 - 31 using Ceric Ions J.H. Trivedi, T.A. Bhatt and H.C. Trivedi Simultaneous equation and absorbance ratio methods for estimation of Fluoxetine 32 - 36 Hydrochloride and Olanzapine in tablet dosage form Vijaykumar K. -
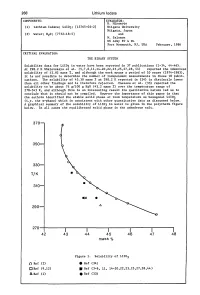
Evaluation of Aqueous Systems
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

P020190719572604117959.Pdf
Springer Series in advanced microelectronics 27 Springer Series in advanced microelectronics Series Editors: K. Itoh T. Lee T. Sakurai W.M.C. Sansen D. Schmitt-Landsiedel The Springer Series in Advanced Microelectronics provides systematic information on all the topics relevant for the design, processing, and manufacturing of microelectronic devices. The books, each prepared by leading researchers or engineers in their fields, cover the basic and advanced aspects of topics such as wafer processing, materials, device design, device technologies, circuit design, VLSI implementation, and subsys- tem technology. The series forms a bridge between physics and engineering and the volumes will appeal to practicing engineers as well as research scientists. 18 Microcontrollers in Practice By I. Susnea and M. Mitescu 19 Gettering Defects in Semiconductors By V.A. Perevoschikov and V.D. Skoupov 20 Low Power VCO Design in CMOS By M. Tiebout 21 Continuous-Time Sigma-Delta A/D Conversion Fundamentals, Performance Limits and Robust Implementations By M. Ortmanns and F. Gerfers 22 Detection and Signal Processing Technical Realization By W.J. Witteman 23 Highly Sensitive Optical Receivers By K. Schneider and H.K. Zimmermann 24 Bonding in Microsystem Technology By J.A. Dziuban 25 Power Management of Digital Circuits in Deep Sub-Micron CMOS Technologies By S. Henzler 26 High-Dynamic-Range (HDR) Vision Microelectronics, Image Processing, Computer Graphics Editor: B. Hoefflinger 27 Advanced Gate Stacks for High-Mobility Semiconductors Editors: A. Dimoulas, E. Gusev, P.C. McIntyre, and M. Heyns Volumes 1–17 are listed at the end of the book. A. Dimoulas E. Gusev P.C. McIntyre M. -

Technical Program
MATSCITECH.ORG TECHNICAL PROGRAM The leading forum addressing structure, properties, processing and performance across the materials community. MATERIALS SCIENCE & TECHNOLOGY OCTOBER 14 – 18, 2018 | GREATER COLUMBUS CONVENTION CENTER | COLUMBUS, OHIO, USA Organizers: Sponsored by: MATSCITECH.ORG MS&T 18 MATERIALS SCIENCE & TECHNOLOGY Date StartTime Room Page PROGRAM HIGHLIGHTS Union MS&T18 Plenary Session TUE AM 55 Station B MS&T18 Poster Session TUE AM & PM Hall A 128 ACerS Basic Science Division Robert B. Sosman Lecture WED PM A111/112 99 ACerS Frontiers of Science and Society - Rustum Roy TUE PM A111/112 56 Lecture ACerS GOMD Alfred R. Cooper Award Session TUE PM A115 63 ACerS Richard M. Fulrath Award Symposium MON PM A111/112 35 PROGRAM AT-A-GLANCE ACerS/EPDC: Arthur L. Friedberg Ceramic Engineering MON AM A111/112 15 Tutorial and Lecture Alpha Sigma Mu Lecture MON PM C170 42 ASM Edward DeMille Campbell Memorial Lecture TUE PM C170 61 ASM-IMS Henry Clifton Sorby Lecture TUE PM A212 64 ADDITIVE MANUFACTURING Additive Manufacturing of Composites and Complex Materials III Metals Additive Manufacturing MON AM A222 15 Additive Manufacturing of Metallic Composites MON PM A222 36 Ceramic Additive Manufacturing TUE PM A222 56 Techniques and Applications WED AM A222 75 Polymer Additive Manufacturing WED PM A222 99 Additive Manufacturing of Metals: Microstructure and Material Properties AM Process Parameter - Effects MON AM A215 16 AM Stainless Steels - Microstructure and Properties MON PM A215 36 Properties of AM Components MON PM A214 37