Development of New Ion-Separation Techniques for Short-Lived Nuclides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mit Stier Und Greif Durch Mecklenburg-Vorpommern
Mit Stier und Greif durch Mecklenburg-Vorpommern Eine kleine politische Landeskunde Hallo... ...wir sind Stier und Greif, die Wappentiere des Bundeslandes Mecklenburg-Vorpommern. Der Stier aus Mecklenburg und der vorpommersche Greif. Und auch wenn wir nicht so aussehen, wir sind wie Zwillinge, sind Brüder im Geiste und im Dienste unseres Landes. Eines wunderbaren Landes wohlgemerkt. Ein Land mit zwei der vier größten Seen, der größten Insel und dem schönsten Landtagssitz Deutschlands. Ein Land mit breiten, weißen Ostseestränden und dem leckersten Fisch. Nun ja, ...letzteres ist Geschmackssache. Wir wollen zeigen, wie es funktioniert, unser wunderbares Land. Wir reisen in die Land- kreise, besuchen die Landeshauptstadt und den Landtag. Wir gehen wählen, sprechen über Geld und blicken auf die Wirtschaft. Wir schauen auf die Geschichte, zählen Ämter und Gemeinden und finden mitten im Land eine Grenze, die gar keine ist. Ach, genug geredet. Los geht’s! 1 Ein Bundesland, zwei Geschichten WIEKER TROMPER BODDEN WIEK LIBBEN Die Geschichte Mecklenburg-Vorpommerns Europa beherrschte, siedelten Germanen BREEGER BODDEN ist eigentlich kurz und schnell erzählt. Im (Sueben) an der Ostsee, die damals Suebi- Jahre 1990, als die DDR in den Geschichts- sches Meer hieß. Die Sueben zogen nach M büchern verschwand, wurde es neugegrün- Süden und die Slawen kamen ins Land. Im RO ST det. Es gab freie Wahlen, Schwerin wurde Jahr 995 taucht Mecklenburg das erste Mal ER BREETZER OW BODDEN Landeshauptstadt und der Landtag zog in einer Urkunde auf. Seinen Namen ver- RASS ins Schweriner Schloss. dankt es einer alten slawischen Burg in der Nähe von Wismar: der Michelenburg. Von Tetzitzer GROSSER See Neugegründet? Ja, denn Mecklenburg-Vor- der einst mächtigen Anlage ist heute nur JASMUNDER Sassnitz pommern hatte es schon einmal gegeben. -

Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatograph
applied sciences Article Comparison of the Characteristic Mass Fragmentations of Phenethylamines and Tryptamines by Electron Ionization Gas Chromatography Mass Spectrometry, Electrospray and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry Bo-Hong Chen, Ju-Tsung Liu, Hung-Ming Chen, Wen-Xiong Chen and Cheng-Huang Lin * Department of Chemistry, National Taiwan Normal University, 88 Sec. 4 Tingchow Road, Taipei 11677, Taiwan; [email protected] (B.-H.C.); [email protected] (J.-T.L.); [email protected] (H.-M.C.); [email protected] (W.-X.C.) * Correspondence: [email protected]; Tel.: +886-2-7734-6170; Fax: +886-2-2932-4249 Received: 18 April 2018; Accepted: 19 June 2018; Published: 22 June 2018 Abstract: Characteristic mass fragmentation of 20 phenethylamine/tryptamine standards were investigated and compared by means of matrix assisted laser desorption/time-of-flight mass spectrometry (MALDI/TOFM), gas chromatography–electron ionization–mass spectrometry (GC-EI/MS) and liquid chromatography–electrospray ionization/mass spectrometry (LC-ESI/MS) + methods. As a result, three characteristic peaks ([M] and fragments from the Cβ-Cα bond breakage) were found to be unique and contained information useful in identifying 2C series compounds based on the GC-EI/MS method. We found that the protonated molecular ion ([M+H]+) and two types of fragments produced from the α-cleavage and β-cleavage processes were useful mass spectral information in the rapid screening and confirmation of phenethylamine and tryptamine derivatives when ESI/MS and MALDI/TOFMS methods were applied. This assay was successfully used to determine samples that contain illicit drugs. Keywords: phenethylamine; tryptamine; MALDI/TOFMS; GC-EI/MS; LC-ESI/MS 1. -

Coupling Gas Chromatography to Mass Spectrometry
Coupling Gas Chromatography to Mass Spectrometry Introduction The suite of gas chromatographic detectors includes (roughly in order from most common to the least): the flame ionization detector (FID), thermal conductivity detector (TCD or hot wire detector), electron capture detector (ECD), photoionization detector (PID), flame photometric detector (FPD), thermionic detector, and a few more unusual or VERY expensive choices like the atomic emission detector (AED) and the ozone- or fluorine-induce chemiluminescence detectors. All of these except the AED produce an electrical signal that varies with the amount of analyte exiting the chromatographic column. The AED does that AND yields the emission spectrum of selected elements in the analytes as well. Another GC detector that is also very expensive but very powerful is a scaled down version of the mass spectrometer. When coupled to a GC the detection system itself is often referred to as the mass selective detector or more simply the mass detector. This powerful analytical technique belongs to the class of hyphenated analytical instrumentation (since each part had a different beginning and can exist independently) and is called gas chromatograhy/mass spectrometry (GC/MS). Placed at the end of a capillary column in a manner similar to the other GC detectors, the mass detector is more complicated than, for instance, the FID because of the mass spectrometer's complex requirements for the process of creation, separation, and detection of gas phase ions. A capillary column is required in the chromatograph because the entire MS process must be carried out at very low pressures (~10-5 torr) and in order to meet this requirement a vacuum is maintained via constant pumping using a vacuum pump. -

ZWAB Ausgabe 2016 Journal
Kundenzeitschrift des Zweckverbandes Wasser/Abwasser Boddenküste ZWAB Ausgabe 2016 Journal www.zvwab.de Mitglied in der www.kowamv.de Inhaltsverzeichnis 02 Service 05 Info´s 03 Angaben zum Verband 10 Statistik 04 Aktuelles 11 Trinkwasserqualität Liebe Leser, die Überlegungen zum jährlichen Vorwort in unserem Kundenjournal führen automatisch auch immer wieder zu einer allgemeinen Einschätzung der aktuellen Situation und der zukünftigen Erwartungen. Die langfristigen Ent- wicklungslinien zu erkennen und zu benennen lautet also die Aufgabe. Wenn nachfolgend auf einige Schwerpunkte eingegangen wird, soll dabei nicht vergessen werden, dass die Aufgabenstellung einer sicheren Trinkwasserversor- gung und Schmutzwasserentsorgung im Verbandsgebiet die eigentliche Hauptaufgabe ist. Die Beherrschung dieser Aufgabe allein führt zu vielfältigen planmäßigen und un- planmäßigen Folgevorgängen. Daneben entwickeln sich aber auch immer wieder Schwerpunkte, auf die sich die Kapazitäten des Verbandes und des Betriebes konzen- das Wirtschaften in einem ländlich geprägten Raum und trieren müssen. Unter diesem Aspekt ist die fortführende die rückgängigen Bevölkerungszahlen sind hier nur einige Gestaltung des personellen Umbruchs ein andauernder Stichworte. Alle diese Fragen bedürfen einer gründlichen Schwerpunkt. Mit externer Unterstützung wurde hier in Analyse und verantwortlicher Entscheidungen. den letzten zwei Jahren eine umfangreiche Organisations- untersuchung durchgeführt. Das Ergebnis dieser Untersu- chung wird Eingang in die zukünftige Verbandsausrichtung -

ZWAB Ausgabe 2014 Journal
Kundenzeitschrift des Zweckverbandes Wasser/Abwasser Boddenküste ZWAB Ausgabe 2014 Journal www.zvwab.de Mitglied in der Inhaltsverzeichnis 02 Service 05 Info´s 03 Angaben zum Verband 10 Statistik 04 Aktuelles 11 Trinkwasserqualität www.kowamv.de Neubau der Kläranlage Lubmin Liebe Leser, im letzten Jahr haben wir an gleicher Stelle eine Bilanz zum 20jährigen Bestehen des ZWAB gezogen, aber auch einen Vorausblick auf zukünftige Schwerpunkte gewagt. Benannt haben wir hierzu die Einarbeitung neuer Mitarbeiter, die Festigung der wirtschaftlichen Größenordnung des Ver- bandes und die Einstellung auf einen zunehmenden Alte- rungsprozess der technischen Anlagen. Die vorgenannte Einschätzung liegt ein Jahr zurück und gilt unverändert fort. Die Einarbeitung neuer Mitarbeiter und die Bewältigung des Geschäftsbetriebes mit insgesamt weniger Personal haben zu neuen Abläufen und zu veränderten Herangehensweisen geführt. Dieser Prozess ist noch nicht abgeschlossen. Was die wirtschaftliche Größenordnung des Verbandes betrifft, hat es keine negativen Entwicklungen gegeben. Neu hinzu- In diesem Zusammenhang ist die Fertigstellung und Inbe- gekommen sind Aufgaben im Rahmen einer Verwaltungs- triebnahme der neuen Kläranlage in Lubmin im Frühherbst gemeinschaft für die Bewirtschaftung von Niederschlags- dieses Jahres noch einmal als ein gegenläufiges Ereignis zu wasser in der Gemeinde Brünzow. Die Einstellung auf die betrachten, da die Anlagenalterung bei einer neuen Anlage zunehmende Anlagenalterung kann man unter verschie- erst wieder zeitversetzt in den nächsten 10 – 20 Jahren eine denen Aspekten betrachten. An dieser Stelle sei lediglich größere Rolle spielen wird. auf die technische Vertiefung zur Bestandsdokumentation Wie immer finden Sie auf den weiteren Seiten unseres Kun- über GIS-Systeme (geografisches Informationssystem) und denjournals hierzu aber auch zu anderen Themen ergän- vorbeugende Untersuchungen durch Dichtheitskontrollen zende und hoffentlich auch interessante Informationen. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -

Modern Mass Spectrometry
Modern Mass Spectrometry MacMillan Group Meeting 2005 Sandra Lee Key References: E. Uggerud, S. Petrie, D. K. Bohme, F. Turecek, D. Schröder, H. Schwarz, D. Plattner, T. Wyttenbach, M. T. Bowers, P. B. Armentrout, S. A. Truger, T. Junker, G. Suizdak, Mark Brönstrup. Topics in Current Chemistry: Modern Mass Spectroscopy, pp. 1-302, 225. Springer-Verlag, Berlin, 2003. Current Topics in Organic Chemistry 2003, 15, 1503-1624 1 The Basics of Mass Spectroscopy ! Purpose Mass spectrometers use the difference in mass-to-charge ratio (m/z) of ionized atoms or molecules to separate them. Therefore, mass spectroscopy allows quantitation of atoms or molecules and provides structural information by the identification of distinctive fragmentation patterns. The general operation of a mass spectrometer is: "1. " create gas-phase ions "2. " separate the ions in space or time based on their mass-to-charge ratio "3. " measure the quantity of ions of each mass-to-charge ratio Ionization sources ! Instrumentation Chemical Ionisation (CI) Atmospheric Pressure CI!(APCI) Electron Impact!(EI) Electrospray Ionization!(ESI) SORTING DETECTION IONIZATION OF IONS OF IONS Fast Atom Bombardment (FAB) Field Desorption/Field Ionisation (FD/FI) Matrix Assisted Laser Desorption gaseous mass ion Ionisation!(MALDI) ion source analyzer transducer Thermospray Ionisation (TI) Analyzers quadrupoles vacuum signal Time-of-Flight (TOF) pump processor magnetic sectors 10-5– 10-8 torr Fourier transform and quadrupole ion traps inlet Detectors mass electron multiplier spectrum Faraday cup Ionization Sources: Classical Methods ! Electron Impact Ionization A beam of electrons passes through a gas-phase sample and collides with neutral analyte molcules (M) to produce a positively charged ion or a fragment ion. -

Fundamentals of Biological Mass Spectrometry and Proteomics
Fundamentals of Biological Mass Spectrometry and Proteomics Steve Carr Broad Institute of MIT and Harvard Modern Mass Spectrometer (MS) Systems Orbitrap Q-Exactive Triple Quadrupole Discovery/Global Experiments Targeted MS MS systems used for proteomics have 4 tasks: • Create ions from analyte molecules • Separate the ions based on charge and mass • Detect ions and determine their mass-to-charge • Select and fragment ions of interest to provide structural information (MS/MS) Electrospray MS: ease of coupling to liquid-based separation methods has made it the key technology in proteomics Possible Sample Inlets Syringe Pump Sample Injection Loop Liquid Autosampler, HPLC Capillary Electrophoresis Expansion of the Ion Formation and Sampling Regions Nitrogen Drying Gas Electrospray Atmosphere Vacuum Needle 3- 5 kV Liquid Nebulizing Gas Droplets Ions Containing Solvated Ions Isotopes Most elements have more than one stable isotope. For example, most carbon atoms have a mass of 12 Da, but in nature, 1.1% of C atoms have an extra neutron, making their mass 13 Da. Why do we care? Mass spectrometers “see” the isotope peaks provided the resolution is high enough. If an MS instrument has resolution high enough to resolve these isotopes, better mass accuracy is achieved. Stable isotopes of most abundant elements of peptides Element Mass Abundance H 1.0078 99.985% 2.0141 0.015 C 12.0000 98.89 13.0034 1.11 N 14.0031 99.64 15.0001 0.36 O 15.9949 99.76 16.9991 0.04 17.9992 0.20 Monoisotopic mass and isotopes We use instruments that resolve the isotopes enabling us to accurately measure the monoisotopic mass MonoisotopicMonoisotopic mass; all 12C, mass no 13C atoms corresponds to 13 lowestOne massC atom peak Two 13C atoms Angiotensin I (MW = 1295.6) (M+H)+ = C62 H90 N17 O14 TheWhen monoisotopic the isotopes mass of aare molecule clearly is the resolved sum of the the accurate monoisotopic masses for the massmost abundant isotope of each element present. -
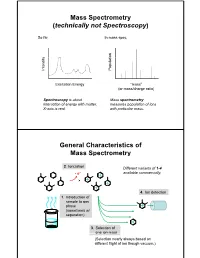
Mass Spectrometry (Technically Not Spectroscopy)
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

An Organic Chemist's Guide to Electrospray Mass Spectrometric
molecules Review An Organic Chemist’s Guide to Electrospray Mass Spectrometric Structure Elucidation Arnold Steckel 1 and Gitta Schlosser 2,* 1 Hevesy György PhD School of Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary; [email protected] 2 Department of Analytical Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary * Correspondence: [email protected] Received: 16 January 2019; Accepted: 8 February 2019; Published: 10 February 2019 Abstract: Tandem mass spectrometry is an important tool for structure elucidation of natural and synthetic organic products. Fragmentation of odd electron ions (OE+) generated by electron ionization (EI) was extensively studied in the last few decades, however there are only a few systematic reviews available concerning the fragmentation of even-electron ions (EE+/EE−) produced by the currently most common ionization techniques, electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). This review summarizes the most important features of tandem mass spectra generated by collision-induced dissociation fragmentation and presents didactic examples for the unexperienced users. Keywords: tandem mass spectrometry; MS/MS fragmentation; collision-induced dissociation; CID; ESI; structure elucidation 1. Introduction Electron ionization (EI), a hard ionization technique, is the method of choice for analyses of small (<1000 Da), nonpolar, volatile compounds. As its name implies, the technique involves ionization by electrons with ~70 eV energy. This energy is high enough to yield very reproducible mass spectra with a large number of fragments. However, these spectra frequently lack the radical type molecular ions (M+) due to the high internal energy transferred to the precursors [1]. -

Vorpommern-Greifswald Können Sie Hier Herunterladen
MIT DEM RAD AUF ENTDECKUNGSTOUR durch die Gutshauslandschaft Vorpommerns Teil II – Vorpommern-Greifswald INHALTSVERZEICHNIS Einführung S. 4 Gutshausrouten Route 1A – Entlang der Dänischen Wiek S. 7 Route 1B – Greifswalder Landschaft S. 8 Route 2 – Beidseits der Tollense S. 15 Route 3 – Nördlich der Peene S. 25 Route 4 – Lassaner Winkel S. 33 Route 5 – Zwischen Achterwasser und Haff S. 41 Route 6 – Landgrabental S. 47 Route 7 – Ueckermünder Heide S. 55 Route 8 – Rund um die Brohmer Berge S. 61 Route 9 – Beidseits der Randow S. 69 Route 10 – Östlich des Randowbruchs S. 75 Literatur S. 82 Informationen S. 83 MIT DEM RAD AUF ENTDECKUNGSTOUR durch die Gutshauslandschaft Vorpommerns Teil II – Vorpommern-Greifswald Einführung Nachdem wir im Jahr 2013 die Radwanderbroschüre zu Guts- und Parkanlagen im Landkreis-Vorpommern-Rügen erstellt haben und diese sich großer Beliebtheit erfreut hat, folgt nun die Fortsetzung mit dem zweiten Teil für den Landkreis Vorpommern- Greifswald. In dieser Broschüre werden elf verschiedene Routen vorgestellt, die dazu einladen, die Guts- und Herrenhäuser und die dazugehörigen Anlagen im Landkreis Vorpommern-Greifswald mit dem Fahrrad zu erkunden. Neben diesen Anlagen sind auch weitere Sehenswürdigkeiten wie bezaubernde Alleen, kleine Dorfkirchen, histo- rische Ortskerne und vor allem die abwechslungsreiche Landschaft mit ihrem unver- wechselbaren vorpommerschen Gepräge zu entdecken. Radeln in Vorpommern ist dank der überwiegend flachen Topographie auch für Unge- übte zu empfehlen. Die leicht hügelige Landschaft macht das Radfahren einfach und die wenigen Steigungen sind gut zu meistern. Zu den einzelnen Routen wurden zwar Empfehlungen zum Richtungsverlauf gegeben, es ist jedoch an windigen Tagen ratsam, die Streckenrichtung vor Ort so zu legen, dass Sie Gegenwind und somit unnötige Anstrengungen vermeiden. -

MV Kulinarisch: Von Usedom Bis Rügen
MVkulinarisch Von Usedom bis Rügen restaurants · cafés · feinkost · hofläden und mehr RIEMS Greifswalder Peenemünder Haken Gristower Wiek KOOS Bodden Freesen- dorfer STRUCK Karrendorf See Spandower- Kooser hagener Wiek Leist See Kölpien- Seebad Spandower- - see Leist III hagen PeenemündeJ Gahlkower Lubmin HL GP O s t s e e Leist II Wampener Haken Wampen Freest B GS Cämmerer Olden- See r hagen Riff Gahlkow r Ostseebad Neuenkirchen Vierow GT Krösliner NSG See NSG Karlshagen E251 B DIE LANCKEN Kröslin GROSSER r Wusterhusen Nonnendorf B WOTIG B96 Loissin B Dänische Kräpelin B B Rubenow Voddow STEINBECKER GREIFSWALD Brünzow VORSTADT Hollendorf Klein Konerow Ostseebad LADEBOW WIECK Ernsthof B Ryck B Trassenheide B B Gustebin Karrin B OSTSEE- Wiek HU VIERTEL Neuendorf Stilow M Ziese Mittelhof O Großer See Kemnitz R Ostseebad ELDENA T SCHÖN- Groß S Ziese Mölschow WALDE II E Zinnowitz Kemnitz Spiegels- Ernsthof Weidehof dorf N Zecherin E Seebad E Ziese B B P Zempin SÜDSTADT SCHÖN- B109 Neu Boltenhagen WALDE I Kemnitzerhagen TANNENKAMP B111 B Mahlzow Bannemin WOLGAST Rieck Netzeband Ostseebad Helms- Weitenhagen Helms- GQ GR JM TORFHEIDE Koserow hagen II Guest Kühlen- Schalense B hagen B Hanshagen hagen B Katzow B Wolgaster Krummin B Fähre V B EICHHOLZ B Potthagen B Karbow B Seebad B96 Jägerhof B111 KARBOWER NEUSTADT Kölpinsee WALD Pritzier Neuendorf Grubenhagen Neeberg Hoher Ort Groß Kiesow Hohendorfer Sauzin Meierei Hohendorf See Krumminer Loddin Hof VII r B Stubben- Ostseebad Ziemitz felde Brand- WRANGELSBURGER B GÖRMITZ Ückeritz WALD P Netzelkow