Rattus Fuscipes) Into Sydney Harbour National Park: Restoring Ecosystem Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Narrabeen Lakes to Manly Lagoon
To NEWCASTLE Manly Lagoon to North Head Personal Care BARRENJOEY and The Spit Be aware that you are responsible for your own safety and that of any child with you. Take care and enjoy your walk. This magnificent walk features the famous Manly Beach, Shelly Beach, and 5hr 30 North Head which dominates the entrance to Sydney Harbour. It also links The walks require average fitness, except for full-day walks which require COASTAL SYDNEY to the popular Manly Scenic Walkway between Manly Cove and The Spit. above-average fitness and stamina. There is a wide variety of pathway alking conditions and terrain, including bush tracks, uneven ground, footpaths, The walk forms part of one of the world’s great urban coastal walks, beaches, rocks, steps and steep hills. Observe official safety, track and road signs AVALON connecting Broken Bay in Sydney’s north to Port Hacking in the south, at all times. Keep well back from cliff edges and be careful crossing roads. traversing rugged headlands, sweeping beaches, lagoons, bushland, and the w Wear a hat and good walking shoes, use sunscreen and carry water. You will Manly Lagoon bays and harbours of coastal Sydney. need to drink regularly, particularly in summer, as much of the route is without Approximate Walking Times in Hours and Minutes 5hr 30 This map covers the route from Manly Lagoon to Manly wharf via North shade. Although cold drinks can often be bought along the way, this cannot to North Head e.g. 1 hour 45 minutes = 1hr 45 Head. Two companion maps, Barrenjoey to Narrabeen Lakes and Narrabeen always be relied on. -

Calaby References
Abbott, I.J. (1974). Natural history of Curtis Island, Bass Strait. 5. Birds, with some notes on mammal trapping. Papers and Proceedings of the Royal Society of Tasmania 107: 171–74. General; Rodents; Abbott, I. (1978). Seabird islands No. 56 Michaelmas Island, King George Sound, Western Australia. Corella 2: 26–27. (Records rabbit and Rattus fuscipes). General; Rodents; Lagomorphs; Abbott, I. (1981). Seabird Islands No. 106 Mondrain Island, Archipelago of the Recherche, Western Australia. Corella 5: 60–61. (Records bush-rat and rock-wallaby). General; Rodents; Abbott, I. and Watson, J.R. (1978). The soils, flora, vegetation and vertebrate fauna of Chatham Island, Western Australia. Journal of the Royal Society of Western Australia 60: 65–70. (Only mammal is Rattus fuscipes). General; Rodents; Adams, D.B. (1980). Motivational systems of agonistic behaviour in muroid rodents: a comparative review and neural model. Aggressive Behavior 6: 295–346. Rodents; Ahern, L.D., Brown, P.R., Robertson, P. and Seebeck, J.H. (1985). Application of a taxon priority system to some Victorian vertebrate fauna. Fisheries and Wildlife Service, Victoria, Arthur Rylah Institute of Environmental Research Technical Report No. 32: 1–48. General; Marsupials; Bats; Rodents; Whales; Land Carnivores; Aitken, P. (1968). Observations on Notomys fuscus (Wood Jones) (Muridae-Pseudomyinae) with notes on a new synonym. South Australian Naturalist 43: 37–45. Rodents; Aitken, P.F. (1969). The mammals of the Flinders Ranges. Pp. 255–356 in Corbett, D.W.P. (ed.) The natural history of the Flinders Ranges. Libraries Board of South Australia : Adelaide. (Gives descriptions and notes on the echidna, marsupials, murids, and bats recorded for the Flinders Ranges; also deals with the introduced mammals, including the dingo). -
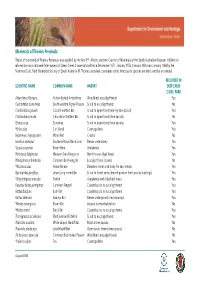
Mammals of Fleurieu Peninsula This List of Mammals of Fleurieu Peninsula Was Applied by the Late P.F
Mammals of Fleurieu Peninsula This list of mammals of Fleurieu Peninsula was applied by the late P.F. Aitken, onetime Curator of Mammals at the South Australian Museum. Additional information was obtained from surveys of Deep Creek Conservation Park in December 1971, January 1972, January 1980 and January 1984 by the Mammal Club, Field Naturalists Society of South Australia (R. Thomas, personal communication). Introduced species are indicated by an asterisk. RECORDED IN SCIENTIFIC NAME COMMON NAME HABITAT DEEP CREEK CONS. PARK Antechinus Flavipes Yellow-footed Antechinus Woodland, eucalypt forest Yes Cercartetus concinnus South western Pigmy Possum Scrub to eucalypt forest No Chalinolobus gouldii Gould's wattled Bat Scrub to open-forest (mainly tree spouts) Yes Chalinolobus moria Chocolate Wattled Bat Scrub to open-forest (tree spouts) No Eptesicus sp. Eptesicus Scrub to open-forest (tree spouts) Yes *Felis calus Cat (feral) Cosmopolitan Yes Hydromys chrysogaster Water Rat Creeks No Isoodon obesulus Southern Brown Bandicoot Dense understorey Yes *Lepus capensis Brown Hare Grasslands Yes Macropus fuliginosus Western Grey Kangaroo Heath to eucalypt forest Yes Miniopterus schreibersii Common Bent-wing Ba Eucalypt forest (caves) No *Mus musculus House Mouse Disturbed areas and early fire succession Yes Nyctophilus geoffroy Lesser Long-eared Bat Scrub to forest, semi cleared pasture (tree spouts, buildings) Yes *Oryctolagus cuniculus Rabbit Grasslands and disturbed areas Yes Pseudocheirus peregrinus Common Ringtail Coastal scrub to eucalypt -

Natural History of the Eutheria
FAUNA of AUSTRALIA 35. NATURAL HISTORY OF THE EUTHERIA P. J. JARMAN, A. K. LEE & L. S. HALL (with thanks for help to J.H. Calaby, G.M. McKay & M.M. Bryden) 1 35. NATURAL HISTORY OF THE EUTHERIA 2 35. NATURAL HISTORY OF THE EUTHERIA INTRODUCTION Unlike the Australian metatherian species which are all indigenous, terrestrial and non-flying, the eutherians now found in the continent are a mixture of indigenous and exotic species. Among the latter are some intentionally and some accidentally introduced species, and marine as well as terrestrial and flying as well as non-flying species are abundantly represented. All the habitats occupied by metatherians also are occupied by eutherians. Eutherians more than cover the metatherian weight range of 5 g–100 kg, but the largest terrestrial eutherians (which are introduced species) are an order of magnitude heavier than the largest extant metatherians. Before the arrival of dingoes 4000 years ago, however, none of the indigenous fully terrestrial eutherians weighed more than a kilogram, while most of the exotic species weigh more than that. The eutherians now represented in Australia are very diverse. They fall into major suites of species: Muridae; Chiroptera; marine mammals (whales, seals and dugong); introduced carnivores (Canidae and Felidae); introduced Leporidae (hares and rabbits); and introduced ungulates (Perissodactyla and Artiodactyla). In this chapter an attempt is made to compare and contrast the main features of the natural histories of these suites of species and, where appropriate, to comment on their resemblance to or difference from the metatherians. NATURAL HISTORY Ecology Diet. The native rodents are predominantly omnivorous. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Current Walks Program - for PDF Download
Current Walks Program - for PDF download See end of this program for Search & Rescue information. Note: Trips recently added or changed are shown in bold. Click here to download as PDF Jan 7 (Tue) OATLEY PARK Mortdale station to Oatley Park; walk around the wetlands, Lime Kiln Bay, Jew Fish Bay with the possibility of a swim in the river, return across the top and back to the station. A delightful spot on the Georges River. DISTANCE: SHORT. TRIP GRADE: EASY MAPS: Sydney Street Directory. LEADER: UTE FOSTER [email protected] 9559 2363 (H) TRANSPORT: TRAIN Jan 9 (Thu) MEADOWBANK - BOTANY BAY (RETURN) - Cycling Meadowbank, Olympic Park, South Strathfield, Cooks River Cyclway, Botany Bay & return. Time for a swim before lunch!. Please ring to confirm details etc. Helmets, Hi-Vis jackets, sunscreen, water, spare tube, pump & repair kit required. Party limit 8. DISTANCE: MEDIUM. TRIP GRADE: MEDIUM MAPS: Street Directory. LEADER: COL HALPIN 98761685 (H). Ring by Tuesday TRANSPORT: Bike Jan 14 (Tue) LANE COVE (q) Riverview, Tambourine Bay, Longueville, Woodford Bay, Northwood, Gore Creek Reserve, Shell Park, Greenwich Point, Greenwich Baths, Smoothey Park, Wollstoncraft Station. Swim at Greenwich Baths (High tide). DISTANCE: MEDIUM. TRIP GRADE: EASY/MEDIUM MAPS: STEP. LEADER: PHIL LAMBE [email protected] 9712 1925 (H) 0439 934 180 (M) TRANSPORT: Public. Jan 16 (Thu) SEVEN BRIDGES - SYDNEY HARBOUR CIRCUIT - Cycling Epping, Fig Tree Bridge, Tarban Ck Bridge, Gladesville Bridge, Iron Cove Bridge, Anzac Bridge, Pyrmont Bridge, Harbour Bridge & optional back to Epping via Gore Hill cycleway. Please ring to confirm details etc. Helmets, Hi-Vis jackets, sunscreen, water, spare tube, pump & repair kit required. -

Karoo Bush Rat
Otomys unisulcatus – Karoo Bush Rat threats that could cause widespread population decline. However, there are potentially synergistic effects of climate change drying up wetlands and overgrazing/ browsing removing at least part of the plant food and cover that this species relies upon. Such effects on subpopulation trends and population distribution should be monitored. Regional population effects: This species is endemic to the assessment region. Its dispersal abilities are not well known. Subpopulations seem to be patchily distributed at the landscape level, according to the presence of favourable habitats. While it is likely that movements and possibly rescue effects exist between subpopulations, Emmanuel Do Linh San others might be physically and genetically isolated. Regional Red List status (2016) Least Concern Distribution National Red List status (2004) Least Concern This species occurs throughout the semi-arid Succulent Reasons for change No change Karoo and Nama-Karoo of South Africa (Monadjem et al. 2015), specifically in the Eastern, Northern and Western Global Red List status (2016) Least Concern Cape provinces, with some limited occurrence in the TOPS listing (NEMBA) (2007) None Fynbos Biome (Vermeulen & Nel 1988; Figure 1). It may marginally occur in southern Namibia but further surveys CITES listing None are required to confirm this. Regardless, the bulk of the Endemic Yes population occurs in South Africa. Kerley and Erasmus (1992) argued that the lodges built by this species are In southern Africa the Karoo Bush Rat vulnerable to destruction by fire. As a result, they is the only rodent that constructs and occupies hypothesised that this shelter-building strategy is only large, dome-shaped stick nests or “lodges”, viable in the absence of frequent burning, and therefore it generally at the base of bushes. -

Government Gazette of the STATE of NEW SOUTH WALES Number 187 Friday, 28 December 2007
Government Gazette OF THE STATE OF NEW SOUTH WALES Number 187 Friday, 28 December 2007 Published under authority by Communications and Advertising Summary of Affairs FREEDOM OF INFORMATION ACT 1989 Section 14 (1) (b) and (3) Part 3 All agencies, subject to the Freedom of Information Act 1989, are required to publish in the Freedom of Information Government Gazette, an up-to-date Summary of Affairs. The requirements are specified in section 14 of Part 2 of the Freedom of Information Act. The Summary of Affairs has to contain a list of each of the Agency's policy documents, advice on how the agency's most recent Statement of Affairs may be obtained and contact details for accessing this information. The Summaries have to be published by the end of June and the end of December each year and need to be delivered to Communications and Advertising two weeks prior to these dates. CONTENTS LOCAL COUNCILS Page Page Page Armidale Dumaresq Council 429 Gosford City Council 567 Richmond Valley Council 726 Ashfield Municipal Council 433 Goulburn Mulwaree Council 575 Riverina Water County Council 728 Auburn Council 435 Greater Hume Shire Council 582 Rockdale City Council 729 Ballina Shire Council 437 Greater Taree City Council 584 Rous County Council 732 Bankstown City Council 441 Great Lakes Council 578 Shellharbour City Council 736 Bathurst Regional Council 444 Gundagai Shire Council 586 Shoalhaven City Council 740 Baulkham Hills Shire Council 446 Gunnedah Shire Council 588 Singleton Council 746 Bega Valley Shire Council 449 Gwydir Shire Council 592 -

Terrestrial Native Mammals of Western Australia
TERRESTRIALNATIVE MAMMALS OF WESTERNAUSTRALIA On a number of occasionswe have been asked what D as y ce r cus u ist ica ud q-Mul Aara are the marsupialsof W.A. or what is the scientiflcname Anlechinusfla.t,ipes Matdo given to a palticular animal whosecommon name only A n t ec h i nus ap i ca I i s-Dlbbler rs known. Antechinusr osemondae-Little Red Antechinus As a guide,the following list of62 speciesof marsupials A nteclt itus mqcdonneIlens is-Red-eared Antechi nus and 59 speciesof othersis publishedbelow. Antechinus ? b ilar n i-Halney' s Antechinus Antec h in us mqculatrJ-Pismv Antechinus N ingaui r idei-Ride's Nirfaui - MARSUPALIA Ningauirinealvi Ealev's-KimNinsaui Ptaiigole*fuilissima beiiey Planigale Macropodidae Plani gale tenuirostris-Narrow-nosed Planigate Megaleia rufa Red Kangaroo Smi nt hopsis mu rina-Common Dulnart Macropus robustus-Etro Smin t hop[is longicaudat.t-Long-tailed Dunnart M acr opus fu Ii g inos,s-Western Grey Kangaroo Sminthops is cras sicaudat a-F at-tailed Dunnart Macrcpus antilo nus Antilope Kangaroo S-nint hopsi s froggal//- Larapinla Macropu"^agi /rs Sandy Wallaby Stnintllopsirgranuli,oer -Whire-railed Dunnart Macrcpus rirra Brush Wallaby Sninthopsis hir t ipes-Hairy -footed Dunnart M acro ptrs eugenii-T ammar Sminthopsiso oldea-^f r oughton's Dunnart Set oni x brac ltyuru s-Quokka A ntec h inomys lanrger-Wuhl-Wuhl On y ch oga I ea Lng uife r a-Kar r abul M.yr nte c o b ius fasc ialrls-N umbat Ony c hogalea Iunq ta-W \rrur.g Notoryctidae Lagorchest es conspic i Ilat us,Spectacied Hare-Wallaby Notorlctes -

Draft Plans of Management for Seaforth Oval, Keirle Park and Tania Park
Draft Plans of Management for Seaforth Oval, Keirle Park and Tania Park Corporate Planning and Strategy Division February, 2004 PLANS OF MANAGEMENT FOR SEAFORTH OVAL, KEIRLE PARK AND TANIA PARK TABLE OF CONTENTS EXECUTIVE SUMMARY .............................................................................................. 7 INTRODUCTION................................................................................................................... 7 PROCESS ............................................................................................................................. 8 1 INTRODUCTION...................................................................................................... 10 1.1 BACKGROUND ........................................................................................................ 10 1.2 LAND TO WHICH THIS PLAN OF MANAGEMENT APPLIES ..................................... 10 1.3 OBJECTIVES OF THIS PLAN OF MANAGEMENT..................................................... 11 1.4 PROCESS OF PREPARING THIS PLAN OF MANAGEMENT....................................... 12 1.4.1 GENERAL PROCESS ............................................................................................ 12 1.4.2 ENVIRONMENTAL ASSESSMENTS ..................................................................... 13 1.4.3 LANDSCAPE MASTERPLANS .............................................................................. 13 1.5 CONTENTS OF THESE PLANS OF MANAGEMENT................................................... 13 2 PLANNING CONTEXT -

Fitz-Stirling 2007-2017 Ten-Year Evaluation Review
Fitz-Stirling 2007-2017 Ten-year Evaluation Review Feb / 2018 P a g e | 1 Acknowledgements: This report has benefited greatly from the discussion and guidance on content, presentation and editing by Annette Stewart, Clair Dougherty and Simon Smale. Their expert assistance is greatly appreciated. Volunteers have played a major and vital role in the monitoring and survey program over the past 5 years and I thank all of those involved. Special thanks go to Dr Sandra Gilfillan for her continuing dedication to the wallaby monitoring and research program. Volunteers Aaron Gove, who provided the bird data analysis and Richard Thomas, who provided the bat data analysis, have made a large contribution to this report and I thank them. I sincerely thank Bill and Jane Thompson who have regularly carried out all the pool monitoring for several years. Thanks also to Barry Heydenrych, Greening Australia, who provided restoration data. Funding to assist the monitoring program and UAV surveys during 2015 was gratefully received from South Coast NRM as part of the Australian Government funded ‘Restoring Gondwana’ program. Funding vital for wallaby monitoring and research was provided by the Diversicon Foundation. Citation: Sanders, A. (2018). Fitz-Stirling 2007-2017 ten-year evaluation review. Unpublished report for Bush Heritage Australia. P a g e | 2 Contents Overview of Fitz-Stirling Project ........................................................................................................ 6 This report evaluates our conservation impact ................................................................................. -

Native Plants of Sydney Harbour National Park: Historical Records and Species Lists, and Their Value for Conservation Monitoring
Native plants of Sydney Harbour National Park: historical records and species lists, and their value for conservation monitoring Doug Benson National Herbarium of New South Wales, Royal Botanic Gardens, Mrs Macquaries Rd, Sydney 2000 AUSTRALIA [email protected] Abstract: Sydney Harbour National Park (lat 33° 53’S; long 151° 13’E), protects significant vegetation on the harbour foreshores close to Sydney City CBD; its floristic abundance and landscape beauty has been acknowledged since the writings of the First Fleet in 1788. Surprisingly, although historical plant collections were made as early as1802, and localised surveys have listed species for parts of the Park since the 1960s, a detailed survey of the flora of whole Park is still needed. This paper provides the first definitive list of the c.400 native flora species for Sydney Harbour National Park (total area 390 ha) showing occurrence on the seven terrestrial sub-regions or precincts (North Head, South Head, Dobroyd Head, Middle Head, Chowder Head, Bradleys Head and Nielsen Park). The list is based on historical species lists, records from the NSW Office of Environment and Heritage (formerly Dept of Environment, Climate Change and Water) Atlas, National Herbarium of New South Wales specimen details, and some additional fieldwork. 131 species have only been recorded from a single precinct site and many are not substantiated with a recent herbarium specimen (though there are historical specimens from the general area for many). Species reported in the sources but for which no current or historic specimen exists are listed separately as being of questionable/non-local status.