Factors Influencing the Distribution of Bush Rats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PUBLISHER S Candolle Herbarium
Guide ERBARIUM H Candolle Herbarium Pamela Burns-Balogh ANDOLLE C Jardin Botanique, Geneva AIDC PUBLISHERP U R L 1 5H E R S S BRILLB RI LL Candolle Herbarium Jardin Botanique, Geneva Pamela Burns-Balogh Guide to the microform collection IDC number 800/2 M IDC1993 Compiler's Note The microfiche address, e.g. 120/13, refers to the fiche number and secondly to the individual photograph on each fiche arranged from left to right and from the top to the bottom row. Pamela Burns-Balogh Publisher's Note The microfiche publication of the Candolle Herbarium serves a dual purpose: the unique original plants are preserved for the future, and copies can be made available easily and cheaply for distribution to scholars and scientific institutes all over the world. The complete collection is available on 2842 microfiche (positive silver halide). The order number is 800/2. For prices of the complete collection or individual parts, please write to IDC Microform Publishers, P.O. Box 11205, 2301 EE Leiden, The Netherlands. THE DECANDOLLEPRODROMI HERBARIUM ALPHABETICAL INDEX Taxon Fiche Taxon Fiche Number Number -A- Acacia floribunda 421/2-3 Acacia glauca 424/14-15 Abatia sp. 213/18 Acacia guadalupensis 423/23 Abelia triflora 679/4 Acacia guianensis 422/5 Ablania guianensis 218/5 Acacia guilandinae 424/4 Abronia arenaria 2215/6-7 Acacia gummifera 421/15 Abroniamellifera 2215/5 Acacia haematomma 421/23 Abronia umbellata 221.5/3-4 Acacia haematoxylon 423/11 Abrotanella emarginata 1035/2 Acaciahastulata 418/5 Abrus precatorius 403/14 Acacia hebeclada 423/2-3 Acacia abietina 420/16 Acacia heterophylla 419/17-19 Acacia acanthocarpa 423/16-17 Acaciahispidissima 421/22 Acacia alata 418/3 Acacia hispidula 419/2 Acacia albida 422/17 Acacia horrida 422/18-20 Acacia amara 425/11 Acacia in....? 423/24 Acacia amoena 419/20 Acacia intertexta 421/9 Acacia anceps 419/5 Acacia julibross. -

Cunninghamia Date of Publication: February 2020 a Journal of Plant Ecology for Eastern Australia
Cunninghamia Date of Publication: February 2020 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) The Australian paintings of Marianne North, 1880–1881: landscapes ‘doomed shortly to disappear’ John Leslie Dowe Australian Tropical Herbarium, James Cook University, Smithfield, Qld 4878 AUSTRALIA. [email protected] Abstract: The 80 paintings of Australian flora, fauna and landscapes by English artist Marianne North (1830-1890), completed during her travels in 1880–1881, provide a record of the Australian environment rarely presented by artists at that time. In the words of her mentor Sir Joseph Dalton Hooker, director of Kew Gardens, North’s objective was to capture landscapes that were ‘doomed shortly to disappear before the axe and the forest fires, the plough and the flock, or the ever advancing settler or colonist’. In addition to her paintings, North wrote books recollecting her travels, in which she presented her observations and explained the relevance of her paintings, within the principles of a ‘Darwinian vision,’ and inevitable and rapid environmental change. By examining her paintings and writings together, North’s works provide a documented narrative of the state of the Australian environment in the late nineteenth- century, filtered through the themes of personal botanical discovery, colonial expansion and British imperialism. Cunninghamia (2020) 20: 001–033 doi: 10.7751/cunninghamia.2020.20.001 Cunninghamia: a journal of plant ecology for eastern Australia © 2020 Royal Botanic Gardens and Domain Trust www.rbgsyd.nsw.gov.au/science/Scientific_publications/cunninghamia 2 Cunninghamia 20: 2020 John Dowe, Australian paintings of Marianne North, 1880–1881 Introduction The Marianne North Gallery in the Royal Botanic Gardens Kew houses 832 oil paintings which Marianne North (b. -

Calaby References
Abbott, I.J. (1974). Natural history of Curtis Island, Bass Strait. 5. Birds, with some notes on mammal trapping. Papers and Proceedings of the Royal Society of Tasmania 107: 171–74. General; Rodents; Abbott, I. (1978). Seabird islands No. 56 Michaelmas Island, King George Sound, Western Australia. Corella 2: 26–27. (Records rabbit and Rattus fuscipes). General; Rodents; Lagomorphs; Abbott, I. (1981). Seabird Islands No. 106 Mondrain Island, Archipelago of the Recherche, Western Australia. Corella 5: 60–61. (Records bush-rat and rock-wallaby). General; Rodents; Abbott, I. and Watson, J.R. (1978). The soils, flora, vegetation and vertebrate fauna of Chatham Island, Western Australia. Journal of the Royal Society of Western Australia 60: 65–70. (Only mammal is Rattus fuscipes). General; Rodents; Adams, D.B. (1980). Motivational systems of agonistic behaviour in muroid rodents: a comparative review and neural model. Aggressive Behavior 6: 295–346. Rodents; Ahern, L.D., Brown, P.R., Robertson, P. and Seebeck, J.H. (1985). Application of a taxon priority system to some Victorian vertebrate fauna. Fisheries and Wildlife Service, Victoria, Arthur Rylah Institute of Environmental Research Technical Report No. 32: 1–48. General; Marsupials; Bats; Rodents; Whales; Land Carnivores; Aitken, P. (1968). Observations on Notomys fuscus (Wood Jones) (Muridae-Pseudomyinae) with notes on a new synonym. South Australian Naturalist 43: 37–45. Rodents; Aitken, P.F. (1969). The mammals of the Flinders Ranges. Pp. 255–356 in Corbett, D.W.P. (ed.) The natural history of the Flinders Ranges. Libraries Board of South Australia : Adelaide. (Gives descriptions and notes on the echidna, marsupials, murids, and bats recorded for the Flinders Ranges; also deals with the introduced mammals, including the dingo). -

Bush Foods and Fibres
Australian Plants Society NORTH SHORE GROUP Ku-ring-gai Wildflower Garden Bush foods and fibres • Plant-based bush foods, medicines and poisons can come from nectar, flowers, fruit, leaves, bark, stems, sap and roots. • Plants provide fibres and materials for making many items including clothes, cords, musical instruments, shelters, tools, toys and weapons. • A fruit is the seed-bearing structure of a plant. • Do not eat fruits that you do not know to be safe to eat. Allergic reactions or other adverse reactions could occur. • We acknowledge the Traditional Custodians of this land and pay our respects to the Elders both past, present and future for they hold the memories, traditions, culture and hope of their people. Plants as food: many native plants must be processed before they are safe to eat. Flowers, nectar, pollen, Sugars, vitamins, honey, lerps (psyllid tents) minerals, starches, manna (e.g. Ribbon Gum proteins & other nutrients Eucalyptus viminalis exudate), gum (e.g. Acacia lerp manna decurrens) Fruit & seeds Staple foods Carbohydrates (sugars, starches, fibre), proteins, fats, vitamins Leaves, stalks, roots, apical Staple foods Carbohydrates, protein, buds minerals Plants such as daisies, lilies, orchids and vines Tubers, rhyzomes were a source of starchy tubers known as Carbohydrate, fibre, yams. The yam daisy Microseris lanceolata protein, vitamins, (Asteraceae) was widespread in inland NSW minerals and other states. The native yam Dioscorea transversa grows north from Stanwell Tops into Qld and Northern Territory and can be eaten raw or roasted as can those of Trachymene incisa. 1 Plant Description of food Other notes Acacia Wattle seed is a rich source of iron, Saponins and tannins and other essential elements. -
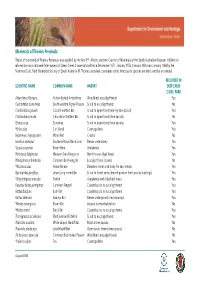
Mammals of Fleurieu Peninsula This List of Mammals of Fleurieu Peninsula Was Applied by the Late P.F
Mammals of Fleurieu Peninsula This list of mammals of Fleurieu Peninsula was applied by the late P.F. Aitken, onetime Curator of Mammals at the South Australian Museum. Additional information was obtained from surveys of Deep Creek Conservation Park in December 1971, January 1972, January 1980 and January 1984 by the Mammal Club, Field Naturalists Society of South Australia (R. Thomas, personal communication). Introduced species are indicated by an asterisk. RECORDED IN SCIENTIFIC NAME COMMON NAME HABITAT DEEP CREEK CONS. PARK Antechinus Flavipes Yellow-footed Antechinus Woodland, eucalypt forest Yes Cercartetus concinnus South western Pigmy Possum Scrub to eucalypt forest No Chalinolobus gouldii Gould's wattled Bat Scrub to open-forest (mainly tree spouts) Yes Chalinolobus moria Chocolate Wattled Bat Scrub to open-forest (tree spouts) No Eptesicus sp. Eptesicus Scrub to open-forest (tree spouts) Yes *Felis calus Cat (feral) Cosmopolitan Yes Hydromys chrysogaster Water Rat Creeks No Isoodon obesulus Southern Brown Bandicoot Dense understorey Yes *Lepus capensis Brown Hare Grasslands Yes Macropus fuliginosus Western Grey Kangaroo Heath to eucalypt forest Yes Miniopterus schreibersii Common Bent-wing Ba Eucalypt forest (caves) No *Mus musculus House Mouse Disturbed areas and early fire succession Yes Nyctophilus geoffroy Lesser Long-eared Bat Scrub to forest, semi cleared pasture (tree spouts, buildings) Yes *Oryctolagus cuniculus Rabbit Grasslands and disturbed areas Yes Pseudocheirus peregrinus Common Ringtail Coastal scrub to eucalypt -

Natural History of the Eutheria
FAUNA of AUSTRALIA 35. NATURAL HISTORY OF THE EUTHERIA P. J. JARMAN, A. K. LEE & L. S. HALL (with thanks for help to J.H. Calaby, G.M. McKay & M.M. Bryden) 1 35. NATURAL HISTORY OF THE EUTHERIA 2 35. NATURAL HISTORY OF THE EUTHERIA INTRODUCTION Unlike the Australian metatherian species which are all indigenous, terrestrial and non-flying, the eutherians now found in the continent are a mixture of indigenous and exotic species. Among the latter are some intentionally and some accidentally introduced species, and marine as well as terrestrial and flying as well as non-flying species are abundantly represented. All the habitats occupied by metatherians also are occupied by eutherians. Eutherians more than cover the metatherian weight range of 5 g–100 kg, but the largest terrestrial eutherians (which are introduced species) are an order of magnitude heavier than the largest extant metatherians. Before the arrival of dingoes 4000 years ago, however, none of the indigenous fully terrestrial eutherians weighed more than a kilogram, while most of the exotic species weigh more than that. The eutherians now represented in Australia are very diverse. They fall into major suites of species: Muridae; Chiroptera; marine mammals (whales, seals and dugong); introduced carnivores (Canidae and Felidae); introduced Leporidae (hares and rabbits); and introduced ungulates (Perissodactyla and Artiodactyla). In this chapter an attempt is made to compare and contrast the main features of the natural histories of these suites of species and, where appropriate, to comment on their resemblance to or difference from the metatherians. NATURAL HISTORY Ecology Diet. The native rodents are predominantly omnivorous. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Pollination Ecology and Evolution of Epacrids
Pollination Ecology and Evolution of Epacrids by Karen A. Johnson BSc (Hons) Submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy University of Tasmania February 2012 ii Declaration of originality This thesis contains no material which has been accepted for the award of any other degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. Karen A. Johnson Statement of authority of access This thesis may be made available for copying. Copying of any part of this thesis is prohibited for two years from the date this statement was signed; after that time limited copying is permitted in accordance with the Copyright Act 1968. Karen A. Johnson iii iv Abstract Relationships between plants and their pollinators are thought to have played a major role in the morphological diversification of angiosperms. The epacrids (subfamily Styphelioideae) comprise more than 550 species of woody plants ranging from small prostrate shrubs to temperate rainforest emergents. Their range extends from SE Asia through Oceania to Tierra del Fuego with their highest diversity in Australia. The overall aim of the thesis is to determine the relationships between epacrid floral features and potential pollinators, and assess the evolutionary status of any pollination syndromes. The main hypotheses were that flower characteristics relate to pollinators in predictable ways; and that there is convergent evolution in the development of pollination syndromes. -

FNCV Register of Photos
FNCV Register of photos - natural history (FNCVSlideReg is in Library computer: My computer - Local Disc C - Documents and settings - Library) [Square brackets] - added or updated name Slide number Title Place Date Source Plants SN001-1 Banksia marginata Grampians 1974 001-2 Xanthorrhoea australis Labertouche 17 Nov 1974 001-3 Xanthorrhoea australis Anglesea Oct 1983 001-4 Regeneration after bushfire Anglesea Oct 1983 001-5 Grevillea alpina Bendigo 1975 001-6 Glossodia major / Grevillea alpina Maryborough 19 Oct 1974 001-7 Discarded - out of focus 001-8 [Asteraceae] Anglesea Oct 1983 001-9 Bulbine bulbosa Don Lyndon 001-10 Senecio elegans Don Lyndon 001-11 Scaevola ramosissima (Hairy fan-flower) Don Lyndon 001-12 Brunonia australis (Blue pincushion) Don Lyndon 001-13 Correa alba Don Lyndon 001-14 Correa alba Don Lyndon 001-15 Calocephalus brownii (Cushion bush) Don Lyndon 001-16 Rhagodia baccata [candolleana] (Seaberry saltbush) Don Lyndon 001-17 Lythrum salicaria (Purple loosestrife) Don Lyndon 001-18 Carpobrotus sp. (Pigface in the sun) Don Lyndon 001-19 Rhagodia baccata [candolleana] Inverloch Don Lyndon 001-20 Epacris impressa Don Lyndon 001-21 Leucopogon virgatus (Beard-heath) Don Lyndon 001-22 Stackhousia monogyna (Candles) Don Lyndon 001-23 Correa reflexa (yellow) Don Lyndon 001-24 Prostanthera sp. Don Lyndon Fungi 002-1 Stinkhorn fungus Aseroe rubra Buckety Plains 30/12/1974 Margarey Lester 002-2 Fungi collection: Botany Group excursion Dom Dom Saddle 28 May 1988 002-3 Aleuria aurantia Aug 1966 R&M Jennings Bairnsdale FNC 002-4 -

Epacris Study Group
ASSOCIATION OF SOCIETIES FOR GROWING AUSTRALIAN PLANTS Inc. EPACRIS STUDY GROUP Group Leader: Gwen Elliot, P.O. Box 655 Heathmont Vic. 3135 NEWSLETTER No. XS (ISSN 103 8-6017) Qctaber zaQ4 Greetings as once again we begin to enjoy the longer days of spring-summer and the encouragement this provides for many of our flowering plants. Despite the generally dry conditions many Epacris species are putting on outstanding floral displays. How are you going with your recording of the flowering times of Epacris impressa in your garden, as well as in nearby bushland or in other areas as you travel within Australia? It really is quite an exciting project because together we, as Study Group members, can make a real contribution to the overall understanding of this species, adding to the knowledge and research of botanists who look in detail at the features of the plant under the microscope and in its natural habitat. It iis a species which occurs both atsea-level and at higher altitudes. How are the flowering times affected when highland plants are cultivated at lower altitudes? Are flowering times different when plants fiom New South Wales for example are gvown much further south in soulhern Victoria or Tasmania ? Epacris impressu seems like an excellent species for us to research in this way. If our project is successful we may perhaps be able to continue with looking at the flowering times of other Epacris which are relatively common in cultivation. In case you have misplaced the recording sheet from our October 2003 Newsletter, another is included in this issue. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Comparison of the Cut Test and Tetrazolium Test in Assessing Seed
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year Comparative ecology of rare and common species in a fire-prone system Mark K.J. Ooi University of Wollongong Ooi, Mark K J, Comparative ecology of rare and common species in a fire-prone system, PhD thesis, School of Biological Sciences, University of Wollongong, 2007, http://ro.uow.edu.au.theses/675 This paper is posted at Research Online. http://ro.uow.edu.au/theses/675 Comparative ecology of rare and common species in a fire-prone system A thesis submitted in fulfilment of the requirements for the award of the degree DOCTOR OF PHILOSOPHY from the UNIVERSITY OF WOLLONGONG by MARK K. J. OOI B. Env. Sci., MSc (Hons) SCHOOL OF BIOLOGICAL SCIENCES 2007 Certification I, Mark Ooi, declare that this thesis, submitted in fulfilment of the requirements for the award of Doctor of Philosophy, in the School of Biological Sciences, University of Wollongong, is wholly my own work unless otherwise referenced or acknowledged. The document has not been submitted for qualifications at any other academic institution. Mark Ooi 30th May 2007 Table of Contents List of Tables.......................................................................................... iv List of Figures......................................................................................... v Abstract................................................................................................... vii Acknowledgements................................................................................