Formating Rules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Nõmme Kohtla- Järve
77 A B C A B C 8 1 1 1 1 Magerburg Y (Suudme) NARVA-JÕESUU ! Kudruküla N a MetskiY Kudruküla r v a Riigiküla Y j Leekovasoo õ AugaY (Vaasa) g 2 2 2 Kudruküla Y i 2 VanakülaY ArukülaY Y Y ! Toila (as) Härmamäe-Vaasovi Sininõmme Ontika-Saka ! Y Saka (as) (Peen-Saka) Vabatküla ! Y " Y Valaste YRahuküla Ontika Toila Martsa YMänniku Meriküla! Y Vepsküla Ontika Y Hoovi ! Kivisaare Vodava ! Saka Saka TOILA ! YOru ! SIIVERTSI YNehatu (Suur-Saka) Kaasikvälja !Tõrvajõe ! ! ! Orujõe ! Kolga-Saka Altküla ! Peeterristi (Sornajõe) ! Y Pühajõe Künnapõhja Päite (as) Puhkova Y Y Suttho Moldova ! (Kärilõpe) ! " Aa " Metsküla Olgina (k) ! Päite Y Y Y Kaasikaia Uikala Türsamäe Y Y (Tünnerküla) ! OLGINA ! " Päite (as) Türsamäe Laagna Põlendiku Y Amula Y VOKA Künnapõhja " MummassaareY Y ! Narva Y YAlulinn Kukruse ! SILLAMÄE Meriküla poolmõis Lapiotsa PÄHKLIMÄE JAANI- Järve Voka Pühajõe (as) P " Vanaküla Y eeslinn KOHTLA- ! ! Paate ü Pimestiku! Udria ! Äkkeküla Y h ! " Suttermu ! Päite! Laagna Tuulukse Tallinna eeslinn (Hiirelinna) a Härmamäe Vanalinn " (*Tõrvassalu) Konju (as) Päite Kannuka " Olgina Peetri eeslinn YLINN Voorepera Y j Y Konju ! Y Mudajõe Y Y ! JÄRVEUuslinn õ !Voka ! ! !Perjatsi YRidaküla Y Soldino Y Kooliküla KUKRUSE ! Pühajõe g (*Kirivere) Aabrami (Väike-Soldina)KadastikuY (IVAN- Y JÄRVE Sinivoore ! Metsamägara i YMoonaküla Vaivina Y Allika Allika Y Kerese ! Aa Pavandu ! Y Vaivina Vaivina Y Sundja ! YY ArukülaY Jaamaküla " Y GOROD) Varja Suitsuküla Y YVanalinn Edise Y Y TulevikuY Paemurru Y Y poolmõis Liivaküla Uus-Sõtke Hiiemetsa ! Uusküla -

1 Katrin Erg Siim Tarros Eesti Geoloogiakeskus OÜ
Katrin Erg Siim Tarros Eesti Geoloogiakeskus OÜ hüdrogeoloogiliste tööde tegevusluba KHY000014 HÜDROGEOLOOGILINE UURING BAARIUMI, ELAVHÕBEDA JA ARSEENI SISALDUSE NING LEVIKU HINDAMISEKS PÕHJAVEEKOGUMITES NR 5, 6, 7 JA 27 Juhatuse liige Aivar Pajupuu Töö finantseeritakse: SA Keskkonnainvesteeringute Keskus „Veemajandus“ programmist. KIK nõukogu 21.03.2017 otsus. TPIS töö nr. 17.3.5.9. Tallinn 2017 1 Töö finantseeritakse: SA Keskkonnainvesteeringute Keskus „Veemajandus“ programmist. KIK nõukogu 21.03.2017 otsus. TPIS töö nr. 17.3.5.9. „Hüdrogeoloogiline uuring baariumi, elavhõbeda ja arseeni sisalduse ning leviku hindamiseks põhjaveekogumites nr 5, 6, 7 ja 27“ ANNOTATSIOON Katrin Erg, Siim Tarros. Hüdrogeoloogiline uuring baariumi, elavhõbeda ja arseeni sisalduse ning leviku hindamiseks põhjaveekogumites nr 5, 6, 7 ja 27. OÜ Eesti Geoloogiakeskus, hüdrogeoloogiaosakond. Tallinn, 2017. Tekst 81 lk, sh 20 joonist, 23 tabelit ja 1 tekstilisa. Keskkonnaministeerium, EGF. Käesolev uuringutöö on koostatud vastavalt Keskkonnaministeeriumi korraldatud riigihanke „Hüdrogeoloogiline uuring baariumi, elavhõbeda ja arseeni sisalduse ning leviku hindamiseks põhjaveekogumites nr 5, 6, 7 ja 27“ (viitenumber 189871) dokumentidele ja töövõtulepingule 4-1/17/173 (EGK 40-85), mis on sõlmitud 04.10.2017. Töö eesmärk on põlevkivi kaevandamisest mõjutatud halvas ja ohustatud seisundis põhjavee- kogumites nr 5, 6, 7 ja 27 baariumi-, elavhõbeda- ja arseenisisalduse põhjuste ja leviku ulatuse ning taustatasemete esmane määramine olemasolevate andmete põhjal. Erinevate uuringute ja seirete käigus kogutud keemilised analüüsid koondati ja koostati andmebaas edasiste detailsemate uuringute jaoks. Põhjaveekogumi seisund on hea, kui ühe kriteeriumina puuduvad ohtlikud ained, sh arseen, kaadmium, plii, elavhõbe, trikloroetüleen, tetrakloroetüleen, sünteetilised ained, või nende kontsentratsioon ei ületa ohtlike ainete põhjavee kvaliteedi piirväärtusi või kui nende ohtlike ainete põhjavees esinemise korral on kindlaks tehtud nende ainete looduslik päritolu. -

Virumaa Hiied
https://doi.org/10.7592/MT2017.66.kaasik Virumaa hiied Ahto Kaasik Teesid: Hiis on ajalooline looduslik pühapaik, millega seostub ohverdamisele, pühakspidamisele, ravimisele, palvetamisele või muule usulisele või taialisele tegevusele viitavaid pärimuslikke andmeid. Üldjuhul on hiis küla pühapaik, rahvapärimuse järgi olevat varem olnud igal külal oma hiis. Samas on mõnda hiiepaika kasutanud terve kihelkond. Artiklis on vaatluse all Virumaa pühapaigad ning ära on toodud Virumaal praeguseks teada olevate hiite nimekiri. Märksõnad: hiis, looduslik pühapaik, Virumaa Eestis on ajalooliste andmete põhjal teada ligikaudu 800 hiit, neist ligi kuuendik Virumaal. Arvestades, et andmed hiitest on jõudnud meieni läbi aastasadade täis sõdu, taude, otsest hävitamist ja ärakeelamist ning usundilise maailmapildi muutumist, on see aukartustäratav hulk. Hiis ühendab kogukonda ja laiemalt rahvast. Hiis täidab õige erinevaid ülesandeid ning on midagi enamat kui looduskaitseala, kooskäimis- või tantsu- koht, vallamaja, haigla, kalmistu, kirik, kohtumaja, kindlus või ohvrikoht. Hiie suhtes puudub tänapäeval kohane võrdlus. Hiis on hiis. Ajalooliste looduslike pühapaikade hulgas moodustavad hiied eraldi rühma. Samma küla Tamme- aluse hiide on rahvast mäletamistmööda kogunenud kogu Mahu (Viru-Nigula) kihelkonnast (Kaasik 2001; Maran 2013). Hiienimelised paigad on ajalooliselt levinud peamiselt põhja pool Tartu – Viljandi – Pärnu joont (Valk 2009: 50). Lõuna pool võidakse sarnaseid pühapai- kasid nimetada kergo-, kumarus-, pühä-, ahi- vm paigaks. Kuid ka Virumaal ei nimetata hiiesarnaseid paiku alati hiieks. Selline on näiteks Lavi pühapaik. Hiietaolisi pühapaikasid leidub meie lähematel ja kaugematel hõimurah- vastel. Sarnased on ka pühapaikadega seotud tõekspidamised ja tavad. Nõnda annavad hiied olulise tähendusliku lisamõõtme meie kuulumisele soome-ugri http://www.folklore.ee/tagused/nr66/kaasik.pdf Ahto Kaasik rahvaste perre. Ja see pole veel kõik. -

Alutaguse Valla Üldplaneering
Registrikood 10171636 Riia 35, Tartu 50410 Tel 730 0310 [email protected] TÖÖ NR 2018-048 Asukoht (L-Est’97) X 6562090 Y 691635 Alutaguse valla üldplaneering Lisad Objekti aadress: IDA-VIRUMAA, ALUTAGUSE VALD Tellija: ALUTAGUSE VALLAVALITSUS Töö täitja: Kobras AS Juhataja: URMAS URI Projektijuht/planeerija: TEELE NIGOLA Volitatud maastikuarhitekt, tase 7 Volitatud ruumilise keskkonna planeerija, tase 7 Kartograaf, planeerija: PIIA KIRSIMÄE Kontrollis: ENE KÖND Aprill 2020 TARTU Alutaguse valla üldplaneering Lisad SISUKORD LISA 1. KSH ARUANNE ....................................................................................................................................... 3 LISA 2. ALUTAGUSE VALLAS ASUVAD SADAMAD .................................................................................... 3 LISA 3. MÄEERALDISED JA MAARDLAD ...................................................................................................... 4 LISA 4. VEEALAD ................................................................................................................................................. 9 LISA 5. KULTUURIVÄÄRTUSLIKUD OBJEKTID ......................................................................................... 17 LISA 6. MUINSUSKAITSEAMETI KOOSTATUD ARHEOLOOGIAPÄRANDI ANALÜÜS JA PROGNOOS ALUTAGUSE VALLAS ................................................................................................................. 24 LISA 7. ETTEPANEK PÄRANDKULTUURI OBJEKTIDEKS ARVAMISEKS ............................................ 33 LISA -

Alajõe, Auvere, Kivinõmme, Kuremäe, Kurtna, Narva, Permisküla, Remniku, Sillamäe, Tamme, Vaivara Ja Voka Jahipiirkonna Moodustamine1
Väljaandja: Keskkonnaminister Akti liik: määrus Teksti liik: algtekst-terviktekst Redaktsiooni jõustumise kp: 21.07.2008 Redaktsiooni kehtivuse lõpp: Hetkel kehtiv Avaldamismärge: RTL 2008, 60, 852 Alajõe, Auvere, Kivinõmme, Kuremäe, Kurtna, Narva, Permisküla, Remniku, Sillamäe, Tamme, Vaivara ja Voka jahipiirkonna moodustamine1 Vastu võetud 10.07.2008 nr 30 Määrus kehtestatakse «Jahiseaduse» § 6 lõike 3 alusel. § 1. Alajõe jahipiirkonna pindala ja piirikirjeldus (1) Alajõe jahipiirkonna pindala on 18 630 hektarit. (2) Alajõe jahipiirkonna piirikirjeldus on järgmine: Alajõe jahipiirkonna piir läheb Tartu–Jõhvi maantee ja Jõuga–Kodamäe maantee ristumiskohast mööda Jõuga– Kodamäe maanteed Tammetaguse kraavini, jätkudes mööda Tammetaguse kraavi Varesmetsa peakraavini ning mööda Varesmetsa peakraavi Armise järve teeni; siitpeale mööda Armise järve teed Iisaku metskonna kvartali PG218 lõunasihini, jätkudes mööda Iisaku metskonna kvartalite PG218, PG219 ja PG220 lõunasihti Piiri sihini; siis mööda Piiri sihti Ongassaare jõeni ning mööda Ongassaare jõge Iisaku metskonna kvartali PG222 läänesihini; sealt mööda Iisaku metskonna kvartalite PG222 lääne- ja lõunasihti, kvartalite PG223, PG224, PG225, PG226, PG227, PG228 ja PG229 lõunasihti Iisaku metskonna kvartali AJ018 loodenurgani; edasi mööda kvartali AJ018 lääne- ja lõunasihti kvartali AJ023 kirdenurgani, jätkudes mööda kvartalite AJ023, AJ033 ja AJ034 idasihti Iisaku metskonna kvartali AJ039 põhjasihini; siit mööda Iisaku metskonna kvartalite AJ039 ja KN175 põhjasihti Mansika teeni ning mööda -

Rahvastiku Ühtlusarvutatud Sündmus- Ja Loendusstatistika
EESTI RAHVASTIKUSTATISTIKA POPULATION STATISTICS OF ESTONIA __________________________________________ RAHVASTIKU ÜHTLUSARVUTATUD SÜNDMUS- JA LOENDUSSTATISTIKA REVIEWED POPULATION VITAL AND CENSUS STATISTICS Ida-Virumaa 1965-1990 Kalev Katus Allan Puur Asta Põldma Aseri Kohtla Lüganuse Toila JÕHVI Sonda Jõhvi Sinimäe Maidla Mäetaguse Illuka Tudulinna Iisaku Alajõe Avinurme Lohusuu Tallinn 2002 EESTI KÕRGKOOLIDEVAHELINE DEMOUURINGUTE KESKUS ESTONIAN INTERUNIVERSITY POPULATION RESEARCH CENTRE RAHVASTIKU ÜHTLUSARVUTATUD SÜNDMUS- JA LOENDUSSTATISTIKA REVIEWED POPULATION VITAL AND CENSUS STATISTICS Ida-Virumaa 1965-1990 Kalev Katus Allan Puur Asta Põldma RU Seeria C No 19 Tallinn 2002 © Eesti Kõrgkoolidevaheline Demouuringute Keskus Estonian Interuniversity Population Research Centre Kogumikuga on kaasas diskett Ida-Virumaa rahvastikuarengut kajastavate joonisfailidega, © Eesti Kõrgkoolidevaheline Demouuringute Keskus. The issue is accompanied by the diskette with charts on demographic development of Ida-Virumaa population, © Estonian Interuniversity Population Research Centre. ISBN 9985-820-66-5 EESTI KÕRGKOOLIDEVAHELINE DEMOUURINGUTE KESKUS ESTONIAN INTERUNIVERSITY POPULATION RESEARCH CENTRE Postkast 3012, Tallinn 10504, Eesti Kogumikus esitatud arvandmeid on võimalik tellida ka elektroonilisel kujul Lotus- või ASCII- formaadis. Soovijail palun pöörduda Eesti Kõrgkoolidevahelise Demouuringute Keskuse poole. Tables presented in the issue on diskettes in Lotus or ASCII format could be requested from Estonian Interuniversity Population Research -

Saagem Tuttavaks – Nemad on ALUTAGUSE TALENDID!
ALUTAGUSE Soovime Alutaguse valla rahvale ilusat Eesti Vabariigi 100. sünnipäeva! nr 2, veebruar 2018 PALJU ÕNNE EESTILE! TASUTA PALJU ÕNNE MEILE KÕIGILE! www.alutagusevald.ee Alutaguse Vallavolikogu ja valla leht Alutaguse Vallavalitsus Saagem tuttavaks – KODULÄVELT MEIE RADA ALGAB Ükski meist ei kasva kodumaata, kodumaata pole ükski mees. Ta kui ema peab meid kõikjal saatma, olema me põues, silme ees. nemad on Kohavad ta kaldad valges vahus, kiigub tuultes ruuge nurmenõlv. ALUTAGUSE TALENDID! Külmad väljad lume karges kahus, laiub põhjamaine taevavõlv. Foto: Kaja Tina Kodulävelt, sealt me rada algab, sealt ta läheb, kaugusesse viib. Pille Nagel „Tu sei“ (Lisette Nagel, Marleen Loik Iisakust), tublid! Muidugi oli üritus ka hästi korraldatud Need me järved, jõed ja merekaldad, Alutaguse talendikonkursi projektijuht „Laevuke läks merele“ (Liisi Kauber Mäetagu- ja kõik sujus ladusalt!“ Kulno Malva: „Tõesti üle lainte tormilinnu tiib. selt), „Jonn“ (Kerto Vinkler Illukalt). Publiku andekad ja mitmekülgsed osalejad, kummar- / M. Kesamaa/ lemmiku tiitli pälvis Marten Nurgamaa Iisakust, dus!“ Piret Päär: „See on väärt üritus, andes 4. veebruaril toimus Iisaku Rahvamajas Alu- kelle etteaste kandis pealkirja „Minu anded“. lastele avaliku esinemise kogemuse ja veel vald- taguse talendikonkurss, mis sai eelmisel aastal Tuhat tänu Iisaku Gümnaasiumi õpilase- konnas, milles nad tunnevad end hästi. Olgu see Õnne Eesti 100! algatatud viie toonase valla – Alajõe, Avinurme, sindusele, kes leidis pingelisel olümpiaadide ja tegevuse valik sündinud kuis tahes, kas õpetaja Kaitsetahet kaitseliitlastele, Iisaku, Illuka ja Tudulinna – koostööprojektina. gümnaasiumi püsimise eest võitlemise perioo- või vanema soovitusel või noore inimese enda rahu, õnne ja edu kodudesse. Seoses valdade liitumisega oli tänavu osavõtjate dil jõudu ja jaksu talendikonkursi korraldami- äratundmisel. Tulla kokku, esineda, tunda ram- ring laiem. -

"EESTI PÕLEVLOODUSVARAD JA -JÄÄTMED" 2012.A (Pdf)
ISSN 1736-0315 Estonian Combustible Natural Resources and Wastes 2012 Eesti Põlevloodusvarad keemia chemistry vääristamine upgrading energeetika energetics ja keskkonnakaitse environmental protection -jäätmed Sindi tehase turbapress või selle analoog. Peat press of Sindi factory, or its analog Kunda-Aru (Kunda-Aro, -Arro) turbaraba plaan. Plan of Kunda-Aru (Kunda-Aro, -Arro) peat bog Eesti turbatööstus 150. Estonian peat industry 150 Lokomotiivi jõul töötav turbapress Pagusoo (Bagu soo) rabas 1920. aastate algul (RMF 665:2). A locomobile powered peat press at Pagusoo raised bog in early 1920 Eesti turbatööstus 150 Eesti Turbaliidu üldkoosolek ja seminar 8. detsember 2011 kujunes Eesti turbatööstuse ajaloos turbaala- ga tegelejatele üheks pikemaks. Tegevuskoht Pärnu hotell Strand. Hommikul kogunesid Eesti Turbaliidu liikmed iga-aastasele üld- koosolekule. Nagu tavaks juhatuse ja tegevjuhi aruanne, majan- dusaasta kokkuvõte, edasiste tegemiste kava ja tootmisandmete kokkuvõte. Eesti Turbaliit ühendab enam kui 30 Eestis turvast kaevanda- vat, töötlevat ja turustavat ettevõtet, lisaks veel turbatootmise ja -töötlemise masinaid valmistavaid ning geoloogiliste uuringute ja projekteerimisega tegelevaid ettevõtteid. Eesti Turbaliitu kuuluvate ettevõtete iga-aastane keskmine turbatoodang on kokku u 4,5–5,0 miljonit m3 eri turbaid, mida eksporditakse ligikaudu sajasse riiki. Siin teeme lühiülevaate üldkoosolekust, seminarist ja ajalookon- verentsist sõnas ja pildis. Eesti Turbaliidu üldkoosolek. Juhatuse esimees Üllar Püvi (OÜ Strenge) aruan- net -

Muraka LKA Ja Muraka Loodusala Püsielupaikade Kaitsekorralduskava 2015-2017
Muraka LKA ja Muraka loodusala püsielupaikade kaitsekorralduskava 2015-2017 1 Sisukord 1. ÜLDISELOOMUSTUS ......................................................................................................5 1.1. Ala iseloomustus .......................................................................................................5 1.2. Maakasutus ..................................................................................................................7 1.3. Huvigrupid...................................................................................................................8 1.4. Kaitsekord ...................................................................................................................9 1.5. Uuritus ....................................................................................................................... 12 1.5.1. Läbiviidud inventuurid ja uuringud ..................................................................... 12 1.5.2. Riiklikud seired ................................................................................................... 12 1.5.3. Inventuuride ja uuringute vajadus ........................................................................ 14 2. Kaitse-eesmärgid, ohutegurid ja vajalikud tegevused ........................................................ 15 2.1. Elustik ....................................................................................................................... 15 2.1.1.Linnud ................................................................................................................ -

Ida-Virumaa Alutaguse Valla Üldplaneeringu Keskkonnamõju Strateegilise Hindamise Aruanne
Registrikood 10171636 Riia 35, Tartu 50410 Tel 730 0310 [email protected] TÖÖ NR 2018-052 Asukoha koordinaadid (L-Est’97) X 6562090 Y 691635 IDA-VIRUMAA ALUTAGUSE VALLA ÜLDPLANEERINGU KESKKONNAMÕJU STRATEEGILISE HINDAMISE ARUANNE AVALIKULE VÄLJAPANEKULE Objekti aadress: IDA-VIRUMAA, ALUTAGUSE VALD Tellija: LUTAGUSE ALLAVALITSUS A V Töö täitja: Kobras AS Juhataja: URMAS URI KSH juhtekspert: URMAS URI KSH juhteksperdi abi, NOEELA KULM keskkonnaekspert: Üldplaneeringu projektijuht / TEELE NIGOLA planeerija: Assistent: PIIA KIRSIMÄE Kontrollis: ENE KÕND Veebruar 2019 TARTU Ida-Virumaa Alutaguse valla üldplaneeringu keskkonnamõju strateegilise hindamise aruanne Üldinfo TÖÖ NIMETUS: Ida-Virumaa Alutaguse valla üldplaneeringu keskkonnamõju strateegilise hindamise aruanne OBJEKTI ASUKOHT: Ida-Virumaa, Alutaguse vald TÖÖ EESMÄRK: Keskkonnamõju strateegilise hindamise läbiviimine Ida-Virumaa Alutaguse valla üldplaneeringule TÖÖ LIIK: Keskkonnamõju strateegiline hindamine TÖÖ TELLIJA: Alutaguse Vallavalitsus Tartu mnt 56, Iisaku alevik 41101 Alutaguse vald Ida-Viru maakond Kontaktisik: Martin Miller keskkonnaspetsialist Tel 336 6916 [email protected] TÖÖ TÄITJA: Kobras AS Registrikood 10171636 Riia 35, 50410 Tartu Tel 730 0310 http://www.kobras.ee KSH juhtekspert: Urmas Uri Tel 730 0310 [email protected] Kontaktisik: Noeela Kulm Tel 730 0310, 5693 9300 [email protected] Ekspertrühm: Urmas Uri – KSH juhtekspert Noeela Kulm – jäätmed, õhk, müra, kaevandused, looduskaitse, maakasutus Rinaldo Rüütli – põhja- ja pinnavesi, inimese tervis ja -

Olulise Ruumilise Mõjuga Aidu Tuulepargi, Seda Toetava Infrastruktuuri Ja Rekreatsioonialade Ning Lasketiiru Asukohavaliku Teemaplaneering Ksh Aruanne
Tellija Maidla Vallavalitsus Dokumendi tüüp KSH aruanne Kuupäev August 2010 Lepingu nr 2009_0034_01 OLULISE RUUMILISE MÕJUGA AIDU TUULEPARGI, SEDA TOETAVA INFRASTRUKTUURI JA REKREATSIOONIALADE NING LASKETIIRU ASUKOHAVALIKU TEEMAPLANEERING KSH ARUANNE olulise ruumilise mõjuga aidu tuulepargi, seda toetava infrastruktuuri ja rekreatsioonialade ning lasketiiru asukohavaliku teemaplaneering ksh Aruanne Versioon 1 Printimise 2010/08/06 kuupäev Koostatud: Kersti Ritsberg, Esta Rahno, Raimo Pajula, Liis Tikerpuu, Heiki Nurmsalu Kontrollitud: Hendrik Puhkim Projekti nr 2009_0034_01 Ramboll Eesti AS Laki 34 12915 Tallinn T +372 664 5808 F +372 664 5818 www.ramboll.ee 2 / 58 olulise ruumilise mõjuga aidu tuulepargi, seda toetava infrastruktuuri ja rekreatsioonialade ning lasketiiru asukohavaliku teemaplaneering ksh Aruanne SISUKORD SISSEJUHATUS ........................................................................................................... 5 1. AIDU TEEMAPLANEERINGU ALA , EESMÄRK JA SEAL KAVANDATAV TEGEVUS. ..... 6 1.1. Aidu teemaplaneeringu ala. ................................................................................... 6 1.2. Aidu teemaplaneeringu eesmärk ............................................................................. 6 1.3. Aidu teemaplaneeringu alal kavandatav tegevus. ...................................................... 7 1.3.1. Sõude- ja veespordikeskus .................................................................................... 7 1.3.2. Tuulepark .......................................................................................................... -
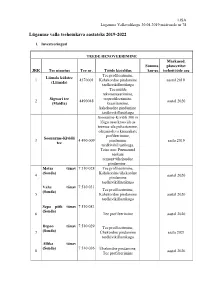
Lüganuse Valla Teehoiukava Aastateks 2019-2022
LISA Lüganuse Vallavolikogu 30.04.2019 määrusele nr 78 Lüganuse valla teehoiukava aastateks 2019-2022 1. Investeeringud TEEDE RENOVEERIMINE Märkused, Summa planeeritav JRK Tee nimetus Tee nr. Tööde kirjeldus km-ga teehoitööde aeg Tee profileerimine, Liimala külatee 1 4370001 Kahekordne pindamine aastal 2019 (Liimala) tardkivikillustikuga Tee mulde rekonstrueerimine, Sigwari tee teeprofileerimine, 2 4490048 aastal 2020 (Maidla) kraavitamine, kahekordne pindamine tardkivikillustikuga Soonurme-Kiviõli 300 m lõigu osas kraavide ja teemaa-ala puhastamine, olemasoleva kruusakate profileerimine, Soonurme-Kiviõli 3 4 490 009 pindamine aasta 2019 tee tardkivikillustikuga. Teise osa: Purunenud teekate remont+ühekordne pindamine . Metsa tänav 7 510 028 Tee profileerimine, (Sonda) Kahekordne/ühekordne 4 aastal 2020 pindamine tardkivikillustikuga Vahe tänav 7 510 031 (Sonda) Tee profileerimine, 5 Kahekordne pindamine aastal 2020 tardkivikillustikuga Sepa põik tänav 7 510 081 (Sonda) 6 Tee profileerimine aastal 2020 Depoo tänav 7 510 029 Tee profileerimine, 7 (Sonda) Ühekordne pindamine aasta 2021 tardkivikillustikuga Allika tänav (Sonda) 7 510 036 Ühekordne pindamine, 8 aastal 2020 Tee profileerimine Uueküla-Sadama Tee profileerimine, 9 tee (mäest alla- 4 370 003 Kahekordne pindamine aastal 2020 Tulivee ristini) tardkivikillustikuga Kippari teeotsani pinnatud, sealt Purtse-Sillaoru tee Ühekordne pindamine 10 4370059 4 867 edasi Sigwarini (Kippar) tardkivikillustikuga tegemata. Aasta 2021 11 Metsa tn (Püssi ) Freesimine,asfalteerimine Aasta 2020/2021