Journal of Natural Science Collections
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View General Report 2004
Lancashire & Cheshire Fauna Society GENERAL REPORT 2004 Edited by Frank Walsh and Dave Bickerton Lancashire & Cheshire Fauna Society Publication No. 105 GENERAL REPORT 2004 Edited by Frank Walsh and Dave Bickerton Lancashire and Cheshire Fauna Society General Report 2004 CONTENTS Introduction ......................................................................................................... F. Walsh .............. 3 Cetaceans in Lancashire, Merseyside, Cheshire and south Cumbria .......... S. J. Hayhow ............. 4 Early records of Polecat, Red Kites & Grass Snakes from North Merseyside ......................................................................... M. E. Greenhalgh ............ 14 Marine Turtles in Lancashire & Cheshire ................................................... S. J. Hayhow ........... 17 The Fylde Natterjack Toad colony ............................... J. Buckley, M. Jones & F. Walsh ............ 20 The Freshwater Fishes of Lancashire, Merseyside & Cheshire ........... M. E. Greenhalgh ............ 23 Recent dragonfly records: with particular reference to the Fylde ...................... F. Walsh ............ 34 The colonization of central and north Lancashire by Comma and Speckled Wood Butterflies ..................................................... J. Wilson & F. Walsh ............ 37 A Checklist of the Macrolepidoptera of Lancashire & Cheshire .................... A. Creaser ........... 45 Rules of the Society ...................................................................................... D. Bickerton -

St Julians Park Species List, 1984 – 2003
St Julians Park Species List, 1984 – 2003 Fungi Species Common Name Date recorded Scleroderma citrinum Common Earth Ball 19/09/99 Amillaria mellea Honey Fungus 19/09/99 Hypholoma sublateridium Brick Caps 19/09/99 Piptoporus belulinus Birch Polypore 19/09/99 Lycoperdon perlatum Common Puffball 19/09/99 Coriolus versicolor Many-Zoned Polypore 19/09/99 Boletus erythropus - 19/09/99 Lactarius quietus Oak/Oily Milk Cap 19/09/99 Russula cyanoxantha The Charcoal Burner 19/09/99 Amanita muscaria Fly Agaric 19/09/99 Laccaria laccata Deceiver 19/09/99 Lepidoptera Species Common Name Date recorded Melanargia galathea Marbled White 1992/3 Venessa cardui Painted Lady 1992/3 Thymelicus sylvestris Small Skipper 1992/3, 06/06/98 Ochlodes venata Large Skipper 1992/3, 06/06/98 Pararge aegeria Speckled Wood 1992/3, 06/06/98 Venessa atalanta Red Admiral 1992/3 Aglais urticae Small Tortoiseshell 1992/3 Polyommatus icarus Common Blue 1992/3, 06/06/98 Pyronia tithonus Gamekeeper 1992/3 Maniola jurtina Meadow Brown 1992/3, 06/06/98 Aphantopus hyperantus Ringlet 1992/3, 06/06/98 Inachis 10 Peacock 1992/3, 23/03/00 Polygonia C-album Comma 1992/3, 23/03/00 Anthocaris cardamines Orange Tip 1992/3 Noctua pronuba Large Yellow Underwing 06/06/98 Pieris brassicae Large White 06/06/98 Zygaena trifolii 5 Spot Burnet 06/06/98 Diboba caeruleocephala Figure of Eight 22/10/99 Xanthia aurago Barred Sallow 22/10/99 Chloroclysta truncate Common Marbled Carpet 22/10/99 Epirrata dilutata November Moth 22/10/99 Epirrata chrysti Pale November Moth 22/10/99, 07/11/99 Chloroclysta -

Acoustic Communication in the Nocturnal Lepidoptera
Chapter 6 Acoustic Communication in the Nocturnal Lepidoptera Michael D. Greenfield Abstract Pair formation in moths typically involves pheromones, but some pyra- loid and noctuoid species use sound in mating communication. The signals are generally ultrasound, broadcast by males, and function in courtship. Long-range advertisement songs also occur which exhibit high convergence with commu- nication in other acoustic species such as orthopterans and anurans. Tympanal hearing with sensitivity to ultrasound in the context of bat avoidance behavior is widespread in the Lepidoptera, and phylogenetic inference indicates that such perception preceded the evolution of song. This sequence suggests that male song originated via the sensory bias mechanism, but the trajectory by which ances- tral defensive behavior in females—negative responses to bat echolocation sig- nals—may have evolved toward positive responses to male song remains unclear. Analyses of various species offer some insight to this improbable transition, and to the general process by which signals may evolve via the sensory bias mechanism. 6.1 Introduction The acoustic world of Lepidoptera remained for humans largely unknown, and this for good reason: It takes place mostly in the middle- to high-ultrasound fre- quency range, well beyond our sensitivity range. Thus, the discovery and detailed study of acoustically communicating moths came about only with the use of electronic instruments sensitive to these sound frequencies. Such equipment was invented following the 1930s, and instruments that could be readily applied in the field were only available since the 1980s. But the application of such equipment M. D. Greenfield (*) Institut de recherche sur la biologie de l’insecte (IRBI), CNRS UMR 7261, Parc de Grandmont, Université François Rabelais de Tours, 37200 Tours, France e-mail: [email protected] B. -

Download (236Kb)
Chapter (non-refereed) Welch, R.C.. 1981 Insects on exotic broadleaved trees of the Fagaceae, namely Quercus borealis and species of Nothofagus. In: Last, F.T.; Gardiner, A.S., (eds.) Forest and woodland ecology: an account of research being done in ITE. Cambridge, NERC/Institute of Terrestrial Ecology, 110-115. (ITE Symposium, 8). Copyright © 1981 NERC This version available at http://nora.nerc.ac.uk/7059/ NERC has developed NORA to enable users to access research outputs wholly or partially funded by NERC. Copyright and other rights for material on this site are retained by the authors and/or other rights owners. Users should read the terms and conditions of use of this material at http://nora.nerc.ac.uk/policies.html#access This document is extracted from the publisher’s version of the volume. If you wish to cite this item please use the reference above or cite the NORA entry Contact CEH NORA team at [email protected] 110 The fauna, including pests, of woodlands and forests 25. INSECTS ON EXOTIC BROADLEAVED jeopardize Q. borealis here. Moeller (1967) suggest- TREES OF THE FAGACEAE, NAMELY ed that the general immunity of Q. borealis to QUERCUS BOREALIS AND SPECIES OF insect attack in Germany was due to its planting in NOTHOFAGUS mixtures with other Quercus spp. However, its defoliation by Tortrix viridana (green oak-roller) was observed in a relatively pure stand of 743 R.C. WELCH hectares. More recently, Zlatanov (1971) published an account of insect pests on 7 species of oak in Bulgaria, including Q. rubra (Table 35), although Since the early 1970s, and following a number of his lists include many pests not known to occur earlier plantings, it seems that American red oak in Britain. -

Additions to the Fauna of Braconidae (Hym., Ichneumonoidea) of Iran Based on the Specimens Housed in Hayk Mirzayans Insect Museum with Six New Records for Iran
J. Ins. Biodivers. Syst. 06(4): 353–364 ISSN: 2423-8112 JOURNAL OF INSECT BIODIVERSITY AND SYSTEMATICS Research Article http://jibs.modares.ac.ir http://zoobank.org/References/F59BDACD-3A4E-42A4-9DE6-4ABA3744048F Additions to the fauna of Braconidae (Hym., Ichneumonoidea) of Iran based on the specimens housed in Hayk Mirzayans Insect Museum with six new records for Iran Ali Ameri1* , Ebrahim Ebrahimi1 & Ali Asghar Talebi2 1 Insect Taxonomy Research Department, Iranian Research Institute of Plant Protection, Agricultural Research Education and Extension Organization (AREEO), Tehran, Islamic Republic of Iran. [email protected]; [email protected] 2 Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, P. O. Box: 14115-336, Tehran, Iran. [email protected] ABSTRACT. This study was based on examination of specimens of the family Braconidae (Hymenoptera: Ichneumonoidea) deposited in Hayk Mirzayans Insect Museum. Totally thirteen species from eleven genera and seven Received: subfamilies, including Braconinae (One genus – One species), Cardiochilinae (1- 02 December, 2019 1), Doryctinae (1-4), Macrocernrinae (1-2) , Opiinae (2-2), Rhyssalinae (1-1), Rogadinae (1-2) were identified, of which six species including Biosteres Accepted: spinaciaeformis Fischer, 1971, Heterospilus rubicola Fischer,1968, Utetes fulvicollis 12 July, 2020 (Thomson, 1895), Aleiodes arcticus (Thomson, 1892), Macrocentrus turkestanicus Published: (Telenga, 1950) and Rhyssalus longicaudis (Tobias & Belokobylskij, 1981) are new 28 July, 2020 records for the Iranian braconid founa. Subject Editor: Ehsan Rakhshani Key words: Taxonomy, Parasitoid wasps, first record Citation: Ameri, A., Ebrahimi, E. & Talebi, A.A. (2020) Additions to the fauna of Braconidae (Hym.: Ichneumonoidea) of Iran based on the specimens housed in Hayk Mirzayans Insect Museum with six new records for Iran. -
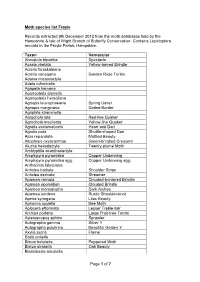
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Impact of Alien Insect Pests on Sardinian Landscape and Culture
Biodiversity Journal , 2012, 3 (4): 297-310 Impact of alien insect pests on Sardinian landscape and culture Roberto A. Pantaleoni 1, 2,* , Carlo Cesaroni 1, C. Simone Cossu 1, Salvatore Deliperi 2, Leonarda Fadda 1, Xenia Fois 1, Andrea Lentini 2, Achille Loi 2, Laura Loru 1, Alessandro Molinu 1, M. Tiziana Nuvoli 2, Wilson Ramassini 2, Antonio Sassu 1, Giuseppe Serra 1, Marcello Verdinelli 1 1Istituto per lo Studio degli Ecosistemi, Consiglio Nazionale delle Ricerche (ISE-CNR), traversa la Crucca 3, Regione Baldinca, 07100 Li Punti SS, Italy; e-mail: [email protected] 2Sezione di Patologia Vegetale ed Entomologia, Dipartimento di Agraria, Università degli Studi di Sassari, via Enrico De Nicola, 07100 Sassari SS, Italy; e-mail: [email protected] *Corresponding author ABSTRACT Geologically Sardinia is a raft which, for just under thirty million years, has been crossing the western Mediterranean, swaying like a pendulum from the Iberian to the Italian Peninsula. An island so large and distant from the other lands, except for its “sister” Corsica, has inevitably developed an autochthonous flora and fauna over such a long period of time. Organisms from other Mediterranean regions have added to this original contingent. These new arrivals were not randomly distributed over time but grouped into at least three great waves. The oldest two correspond with the Messinian salinity crisis about 7 million years ago and with the ice age, when, in both periods, Sardinia was linked to or near other lands due to a fall in sea level. The third, still in progress, is linked to human activity. -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Climatic Condition in Mandi Distric
Research Paper Volume : 4 | Issue : 1 | January 2015 • ISSN No 2277 - 8179 Taxonomic Studies on Light Trap Collected Biotechnology Species of Abraxas Leach Under Agro- KEYWORDS : Taxonomic, Genitalia, Wing, Adeagus Climatic Condition in Mandi District of Himachal Pradesh Forest Protection Division, Himalayan Forest Research Institute, Conifer Campus VIKRANT THAKUR Panthaghati, Shimla Himachal Pradesh 171009 MANOJ KUMAR Faculty of Biotechnology, Shoolini university, Solan HP-172230 ABSTRACT The light trap collection by using mercury lamp indicated that Abraxas Leach under agro-climatic condition of Mandi district of Himachal Pradesh is comprises of three species viz. Abraxas picaria Moore, Abraxas leucostola Hampson and Abraxas sylvata Scopoli. These species obatain their peak in July- August . Abraxas leucostola Hampson was found most abundant followed by Abraxas picaria Moore and Abraxas sylvata Scopoli on the basis of daily catches. The taxonomic study on these spe- cies carried out and described in detail. A key to the species of Abraxas Leach has also been provided for their easy identification. Introduction: DEX (Beccaloni et.al. 2003). The hierarchy of different moth is This moth is mostly white with brownish patches across all of given by Van Nieukerken et al. (2011) the wings. There are small areas of pale gray on the forewings and hind wings. They resemble bird droppings while resting on Result: the upper surface of leaves. The adults fly from late May to early Abraxas picaria Moore August. They are attracted to light. The wingspan is 38 mm. to picariaMoore, 1868, Proc. zool. Soc. Lond. 1893(2): 393 (Abraxas). 48 mm. The moth is nocturnal and is easy to find during the day. -

Desktop Biodiversity Report
Desktop Biodiversity Report Land at Balcombe Parish ESD/14/747 Prepared for Katherine Daniel (Balcombe Parish Council) 13th February 2014 This report is not to be passed on to third parties without prior permission of the Sussex Biodiversity Record Centre. Please be aware that printing maps from this report requires an appropriate OS licence. Sussex Biodiversity Record Centre report regarding land at Balcombe Parish 13/02/2014 Prepared for Katherine Daniel Balcombe Parish Council ESD/14/74 The following information is included in this report: Maps Sussex Protected Species Register Sussex Bat Inventory Sussex Bird Inventory UK BAP Species Inventory Sussex Rare Species Inventory Sussex Invasive Alien Species Full Species List Environmental Survey Directory SNCI M12 - Sedgy & Scott's Gills; M22 - Balcombe Lake & associated woodlands; M35 - Balcombe Marsh; M39 - Balcombe Estate Rocks; M40 - Ardingly Reservior & Loder Valley Nature Reserve; M42 - Rowhill & Station Pastures. SSSI Worth Forest. Other Designations/Ownership Area of Outstanding Natural Beauty; Environmental Stewardship Agreement; Local Nature Reserve; National Trust Property. Habitats Ancient tree; Ancient woodland; Ghyll woodland; Lowland calcareous grassland; Lowland fen; Lowland heathland; Traditional orchard. Important information regarding this report It must not be assumed that this report contains the definitive species information for the site concerned. The species data held by the Sussex Biodiversity Record Centre (SxBRC) is collated from the biological recording community in Sussex. However, there are many areas of Sussex where the records held are limited, either spatially or taxonomically. A desktop biodiversity report from SxBRC will give the user a clear indication of what biological recording has taken place within the area of their enquiry. -

Diversity of the Moth Fauna (Lepidoptera: Heterocera) of a Wetland Forest: a Case Study from Motovun Forest, Istria, Croatia
PERIODICUM BIOLOGORUM UDC 57:61 VOL. 117, No 3, 399–414, 2015 CODEN PDBIAD DOI: 10.18054/pb.2015.117.3.2945 ISSN 0031-5362 original research article Diversity of the moth fauna (Lepidoptera: Heterocera) of a wetland forest: A case study from Motovun forest, Istria, Croatia Abstract TONI KOREN1 KAJA VUKOTIĆ2 Background and Purpose: The Motovun forest located in the Mirna MITJA ČRNE3 river valley, central Istria, Croatia is one of the last lowland floodplain 1 Croatian Herpetological Society – Hyla, forests remaining in the Mediterranean area. Lipovac I. n. 7, 10000 Zagreb Materials and Methods: Between 2011 and 2014 lepidopterological 2 Biodiva – Conservation Biologist Society, research was carried out on 14 sampling sites in the area of Motovun forest. Kettejeva 1, 6000 Koper, Slovenia The moth fauna was surveyed using standard light traps tents. 3 Biodiva – Conservation Biologist Society, Results and Conclusions: Altogether 403 moth species were recorded Kettejeva 1, 6000 Koper, Slovenia in the area, of which 65 can be considered at least partially hygrophilous. These results list the Motovun forest as one of the best surveyed regions in Correspondence: Toni Koren Croatia in respect of the moth fauna. The current study is the first of its kind [email protected] for the area and an important contribution to the knowledge of moth fauna of the Istria region, and also for Croatia in general. Key words: floodplain forest, wetland moth species INTRODUCTION uring the past 150 years, over 300 papers concerning the moths Dand butterflies of Croatia have been published (e.g. 1, 2, 3, 4, 5, 6, 7, 8).