Project-Group ESRF-Beamline (Robl-Erg)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
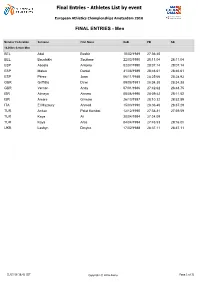
Final Entries - Athletes List by Event
Final Entries - Athletes List by event European Athletics Championships Amsterdam 2016 FINAL ENTRIES - Men Member Federation Surname First Name DoB PB SB 10,000m Senior Men BEL Abdi Bashir 10/02/1989 27:36.40 BEL Bouchikhi Soufiane 22/03/1990 28:11.04 28:11.04 ESP Abadía Antonio 02/07/1990 28:07.14 28:07.14 ESP Mateo Daniel 31/08/1989 28:46.61 28:46.61 ESP Pérez Juan 06/11/1988 28:25.66 28:28.93 GBR Griffiths Dewi 09/08/1991 28:34.38 28:34.38 GBR Vernon Andy 07/01/1986 27:42.62 28:48.75 ISR Almeya Aimeru 08/06/1990 28:09.42 28:11.52 ISR Amare Girmaw 26/10/1987 28:10.32 28:52.89 ITA El Mazoury Ahmed 15/03/1990 28:36.40 28:37.29 TUR $UÕNDQ Polat Kemboi 12/12/1990 27:38.81 27:59.59 TUR Kaya Ali 20/04/1994 27:24.09 TUR Kaya Aras 04/04/1994 27:48.53 29:16.0h UKR Lashyn Dmytro 17/02/1988 28:37.11 28:37.11 01/07/16 16:42 CET Copyright © 2016 Arena Page 1 of 32 Final Entries - Athletes List by event European Athletics Championships Amsterdam 2016 FINAL ENTRIES - Men Member Federation Surname First Name DoB PB SB 100m Senior Men AUT Fuchs Markus 14/11/1995 10.36 10.36 BUL Dimitrov Denis 10/02/1994 10.16 10.18 CZE Veleba Jan 06/12/1986 10.23 10.28 ESP Hortelano Bruno 18/09/1991 10.06 10.06 ESP Rodríguez Ángel David 25/04/1980 10.14 10.24 EST Niit Marek 09/08/1987 10.19 10.30 FRA Dutamby Stuart 24/04/1994 10.12 10.12 FRA Lemaitre Christophe 11/06/1990 9.92 10.09 FRA Vicaut Jimmy 27/02/1992 9.86 9.86 FRA Zeze Mickael-Meba 19/05/1994 10.21 10.21 GBR Edoburun Ojie 02/06/1996 10.16 10.19 GBR Ellington James 06/09/1985 10.11 10.11 GBR Gemili Adam 06/10/1993 -

Ebook As Diasporas Dos Judeus E Cristaos.Pdf
As Diásporas dos Judeus e Cristãos- -Novos de Origem Ibérica entre o Mar Mediterrâneo e o Oceano Atlântico. Estudos ORGANIZAÇÃO : José Alberto R. Silva Tavim Hugo Martins Ana Pereira Ferreira Ângela Sofia Benoliel Coutinho Miguel Andrade Lisboa Centro de História da Universidade de Lisboa 2020 Título As Diásporas dos Judeus e Cristãos-Novos de Origem Ibérica entre o Mar Mediterrâneo e o Oceano Atlântico. Estudos Organização José Alberto R. Silva Tavim, Hugo Martins, Ana Pereira Ferreira, Ângela Sofia Benoliel Coutinho e Miguel Andrade Revisão André Morgado Comissão científica Ana Isabel Lopez-Salazar Codes (U. Complutense de Madrid), Anat Falbel (U. Federal do Rio de Janeiro), Ângela Domingues (U. Lisboa), Beatriz Kushnir (Directora, Arq. Geral da Cidade do Rio de Janeiro), Blanca de Lima (U. Francisco de Miranda, Coro; Acad. Nac. de la Historia-Capítulo Falcón, Venezuela), Claude B. Stuczynski (Bar-Ilan U.), Cynthia Michelle Seton-Rogers (U. of Texas-Dallas), Daniel Strum (U. São Paulo), Daniela Levy (U. São Paulo), Edite Alberto (Dep. Património Cultural/C. M. Lisboa; CHAM, FCSH-UNL), Elvira Azevedo Mea (U. Porto), Eugénia Rodrigues (U. Lisboa), Hernán Matzkevich (U. Purdue), Joelle Rachel Rouchou (Fund. Casa de Rui Barbosa, Rio de Janeiro), Jorun Poettering (Harvard U.), Maria Augusta Lima Cruz (ICS-U. do Minho; CHAM, FCSH, UNL), Maria Manuel Torrão (U. Lisboa), Moisés Orfali (Bar-Ilan U.), Nancy Rozenchan (U. de São Paulo), Palmira Fontes da Costa (U. Nova de Lisboa), Timothy D. Walker (U. Massachusetts Dartmouth) e Zelinda Cohen (Inst. do Património Cultural, Cabo Verde) Capa Belmonte, com Sinagoga Bet Eliahu. Fotografia de José Alberto R. -

Jewish Cemetries, Synagogues, and Mass Grave Sites in Ukraine
Syracuse University SURFACE Religion College of Arts and Sciences 2005 Jewish Cemetries, Synagogues, and Mass Grave Sites in Ukraine Samuel D. Gruber United States Commission for the Preservation of America’s Heritage Abroad Follow this and additional works at: https://surface.syr.edu/rel Part of the Religion Commons Recommended Citation Gruber, Samuel D., "Jewish Cemeteries, Synagogues, and Mass Grave Sites in Ukraine" (2005). Full list of publications from School of Architecture. Paper 94. http://surface.syr.edu/arc/94 This Report is brought to you for free and open access by the College of Arts and Sciences at SURFACE. It has been accepted for inclusion in Religion by an authorized administrator of SURFACE. For more information, please contact [email protected]. JEWISH CEMETERIES, SYNAGOGUES, AND MASS GRAVE SITES IN UKRAINE United States Commission for the Preservation of America’s Heritage Abroad 2005 UNITED STATES COMMISSION FOR THE PRESERVATION OF AMERICA’S HERITAGE ABROAD Warren L. Miller, Chairman McLean, VA Members: Ned Bandler August B. Pust Bridgewater, CT Euclid, OH Chaskel Besser Menno Ratzker New York, NY Monsey, NY Amy S. Epstein Harriet Rotter Pinellas Park, FL Bingham Farms, MI Edgar Gluck Lee Seeman Brooklyn, NY Great Neck, NY Phyllis Kaminsky Steven E. Some Potomac, MD Princeton, NJ Zvi Kestenbaum Irving Stolberg Brooklyn, NY New Haven, CT Daniel Lapin Ari Storch Mercer Island, WA Potomac, MD Gary J. Lavine Staff: Fayetteville, NY Jeffrey L. Farrow Michael B. Levy Executive Director Washington, DC Samuel Gruber Rachmiel -
Jewish Cemeteries, Synagogues, and Mass Grave Sites in Ukraine
JEWISH CEMETERIES, SYNAGOGUES, AND MASS GRAVE SITES IN UKRAINE United States Commission for the Preservation of America’s Heritage Abroad 2005 UNITED STATES COMMISSION FOR THE PRESERVATION OF AMERICA’S HERITAGE ABROAD Warren L. Miller, Chairman McLean, VA Members: Ned Bandler August B. Pust Bridgewater, CT Euclid, OH Chaskel Besser Menno Ratzker New York, NY Monsey, NY Amy S. Epstein Harriet Rotter Pinellas Park, FL Bingham Farms, MI Edgar Gluck Lee Seeman Brooklyn, NY Great Neck, NY Phyllis Kaminsky Steven E. Some Potomac, MD Princeton, NJ Zvi Kestenbaum Irving Stolberg Brooklyn, NY New Haven, CT Daniel Lapin Ari Storch Mercer Island, WA Potomac, MD Gary J. Lavine Staff: Fayetteville, NY Jeffrey L. Farrow Michael B. Levy Executive Director Washington, DC Samuel Gruber Rachmiel Liberman Research Director Brookline, MA Katrina A. Krzysztofiak Laura Raybin Miller Program Manager Pembroke Pines, FL Patricia Hoglund Vincent Obsitnik Administrative Officer McLean, VA 888 17th Street, N.W., Suite 1160 Washington, DC 20006 Ph: ( 202) 254-3824 Fax: ( 202) 254-3934 E-mail: [email protected] May 30, 2005 Message from the Chairman One of the principal missions that United States law assigns the Commission for the Preservation of America’s Heritage Abroad is to identify and report on cemeteries, monuments, and historic buildings in Central and Eastern Europe associated with the cultural heritage of U.S. citizens, especially endangered sites. The Congress and the President were prompted to establish the Commission because of the special problem faced by Jewish sites in the region: The communities that had once cared for the properties were annihilated during the Holocaust. -

A NSF Physics Frontier Center Annual Report 2005-2006
A NSF Physics Frontier Center Annual Report 2005-2006 June 29, 2006 i Table of Contents 1 Executive Summary 1 2 Research Accomplishments and Plans 4 2.a Major Research Accomplishments . 4 2.a.1 Research Highlights . 4 2.a.2 Detailed Research Activities: MRC I- Theory . 5 2.a.3 Detailed Research Activities: MRC II- Structures in the Uni- verse ..................................... 20 2.a.4 Detailed Research Activities: MRC III - Cosmic Radiation Backgrounds ................................ 24 2.a.5 Detailed Research Activities: MRC IV - Particles from Space . 28 2.a.6 References . 32 2.b Research Organizational Details . 33 2.c Plans for the Coming Year . 33 3 Publications, Awards and Technology Transfers 40 3.a List of Publications in Peer Reviewed Journals . 40 3.b List of Publications in Peer Reviewed Conference Proceedings . 48 3.c Invited Talks by Institute Members . 52 3.d Honors and Awards . 56 3.e Technology Transfer . 57 4 Education and Human Resources 58 4.a Graduate and Postdoctoral Training . 58 4.a.1 Research Training . 58 4.a.2 Curriculum Development: . 62 4.b Undergraduate Education . 63 4.b.1 Undergraduate Research Experiences: . 63 4.b.2 Undergraduate Curriculum Development: . 64 4.c Educational Outreach . 65 4.c.1 K-12 Programs: Space Explorers . 66 4.c.2 Web-Based Educational Activities: . 69 4.c.3 Other: . 69 4.d Enhancing Diversity . 72 5 Community Outreach and Knowledge Transfer 74 5.a Visitor Participation in Center . 74 5.a.1 Long term visitors . 74 5.a.2 Short term and seminar visitors . 75 5.b Workshops and Symposia . -

Adopting HIV/AIDS Policy in Russia and South Africa, 1999-2008
Syracuse University SURFACE Maxwell School of Citizenship and Public Political Science - Dissertations Affairs 2011 Treatment as a Common Good: Adopting HIV/AIDS Policy in Russia and South Africa, 1999-2008 Vladislav Kravtsov Syracuse University Follow this and additional works at: https://surface.syr.edu/psc_etd Part of the Political Science Commons Recommended Citation Kravtsov, Vladislav, "Treatment as a Common Good: Adopting HIV/AIDS Policy in Russia and South Africa, 1999-2008" (2011). Political Science - Dissertations. 97. https://surface.syr.edu/psc_etd/97 This Dissertation is brought to you for free and open access by the Maxwell School of Citizenship and Public Affairs at SURFACE. It has been accepted for inclusion in Political Science - Dissertations by an authorized administrator of SURFACE. For more information, please contact [email protected]. ABSTRACT The goal of this dissertation is to increase our understanding of domestic policy responses to initiatives and expertise as provided by international health organizations. Although following international recommendations often improves domestic public health, in certain circumstances resistance to adopting them persists. My core theoretical argument suggests that a strong societal agreement about what constitutes the ―common good‖ served by state (e.g., ―social purpose‖) informs how domestic policy-makers evaluate the benefits of adopting international recommendations. This agreement also affects how governments frame the provision of treatment, decide which subpopulations should benefit from the consumption of public good, and choose their partners in policy implementation. To demonstrate the impact of social purpose I examine how, why and with what consequences Russia and South Africa adopted the external best case practices, guidelines, and recommendations in regard to the HIV/AIDS treatment. -

GCM2017 Catalogo+Calendario V6
ASSOCIAZIONE CULTURALE Kinemathek, Berlin); Anke Mebold (Deutsches “LE GIORNATE DEL CINEMA MUTO” Filminstitut – DIF); Andreas Thein (Filmmuseum Düsseldorf); Stefan Drößler (Filmmuseum Soci fondatori München); Oliver Hanley (Filmuniversität Paolo Cherchi Usai, Lorenzo Codelli, Babelsberg Konrad Wolf); Anke Wilkening Piero Colussi, Andrea Crozzoli, Luciano De (Friedrich-Wilhelm-Murnau-Stiftung); Anna Giusti, Livio Jacob, Carlo Montanaro, Mario Leippe, Reiner Ziegler (Landesfilmsammlung Quargnolo†, Piera Patat, Davide Turconi† Baden-Württemberg). Presidente Giappone: Masaki Daibo, Yoshiro Irie, Akira Livio Jacob Tochigi (National Film Center, Tokyo); Alexander Direttore emerito Jacoby, Johan Nordström David Robinson Italia: Luigi Calabrese (Associazione La Bottega Direttore delle Idee, Taranto); Carmen Accaputo Jay Weissberg (Cineteca di Bologna); Luisa Comencini, Roberto Della Torre, Matteo Pavesi, Marcello Seregni (Fondazione Cineteca Italiana, Milano); Laura Ringraziamo sentitamente per aver collaborato Argento, Daniela Currò, Franca Farina, Felice al programma: Laudadio, Irela Núñez Del Pozo, Maria Assunta Argentina: Fernando Citara (Archivo General de la Pimpinelli (Fondazione CSC - Cineteca Nazionale, Nación, Buenos Aires). Roma); Maria Ida Biggi, Marianna Zannoni (Fondazione Giorgio Cini, Venezia); Immagine Australia: Sally Jackson, Meg Labrum, Gayle Lake, Ritrovata, Bologna; Stella Dagna, Claudia Gianetto Caitlyn Leon (National Film and Sound Archive, (Museo Nazionale del Cinema, Torino); Luca Canberra). Mazzei (Università di Roma -

International Conference Education and Language Edition
INTERNATIONAL CONFERENCE EDUCATION AND LANGUAGE EDITION 19 AUGUST 2019 Novotel, Athens, Greece CONFERENCE PROCEEDINGS BOOK 1 | VOLUME 2 EDUCATION AND EDUCATIONAL RESEARCH LANGUAGE AND LINGUISTICS DISCLAIMER This book contains abstracts, keywords and full papers, which has gone under double blind peer-review by NORDSCI Review Committee. Authors of the articles are responsible for the content and accuracy. The book covers the scientific sections Education and Educational research, Language and Linguistics. Opinions expressed might not necessary affect the position of NORDSCI Committee Members and Scientific Council. Information in the NORDSCI 2019 Conference proceedings is subject to change without any prior notice. No parts of this book can be reproduced or transmitted in any form or by any mean without the written confirmation of the Scientific Council of NORDSCI. Copyright © NORDSCI 2019 All right reserved by NORDSCI International Conference Published by SAIMA CONSULT LTD, Sofia, Bulgaria Total print 60 ISSN 2603-4107 ISBN 978-619-7495-05-8 DOI 10.32008/NORDSCI2019/B1/V2 NORDSCI CONFERENCE Contact person: Maria Nikolcheva e-mail: [email protected] URL: www.nordsci.org SCIENTIFIC PARTNERS OF NORDSCI INTERNATIONAL CONFERENCE EUROPEAN ACADEMY OF SCIENCES, ARTS AND LETTERS SLOVAK ACADEMY OF SCIENCES CZECH ACADEMY OF SCIENCES NATIONAL ACADEMY OF SCIENCES OF UKRAINE BULGARIAN ACADEMY OF SCIENCES POLISH ACADEMY OF SCIENCES ACADEMY OF SCIENCES OF HUNGARY SERBIAN ACADEMY OF SCIENCES TURKISH ACADEMY OF SCIENCES ACADEMY OF SCIENCES OF MOLDOVA ISLAMIC WORLD ACADEMY OF SCIENCES LATVIA ACADEMY OF SCIENCES SCIENTIFIC COMMITTEE ABAY KAIRZHANOV, RUSSIA LAURA PRICOP, ROMANIA ALEXANDER IVANOV, RUSSIA DOROTA ORTENBURGER, POLAND CHIRCU SORINA, ROMANIA JONAS JAKAITIS, LITHUANIA ELENI HADJIKAKOU, GREECE MAGDALENA BALICA, ROMANIA JANA WALDNEROVA, SLOVAKIA TABLE OF CONTENTS Section EDUCATION AND EDUCATIONAL RESEARCH | 1. -

The Ukrainian Weekly 2014, No.6
www.ukrweekly.com INSIDE: l Campaign of terror against Maidan protesters – page 3 l A preview of the Olympic Games in Sochi – page 9 l Photo report: The people of the Maidan – centerfold THEPublished U by theKRAINIAN Ukrainian National Association Inc., a fraternal W non-profit associationEEKLY Vol. LXXXII No. 6 THE UKRAINIAN WEEKLY SUNDAY, FEBRUARY 9, 2014 $1/$2 in Ukraine No solution to Ukraine crisis as proposals diverge by Zenon Zawada KYIV – Ukrainian President Viktor Yanukovych has yet to decide on a solution to Ukraine’s political crisis and he doesn’t have much time left as Ukraine’s economy is in a tailspin. The Russian government decided to freeze its financial aid of $12 billion (3 billion was provided in December) and cheaper natural gas prices the day after Ukrainian Prime Minister Mykola Azarov resigned on January 28. Meanwhile, the hryvnia has fallen the most of any currency this year except the Argentine peso, hitting its lowest value in five years on February 5. “We estimate that National Bank reserves can fall to an alarming level of $15 billion already by the end of February, which can lead to further hryvnia devaluation beyond the Bank’s control, further cuts to Ukraine’s credit ratings and eventually lead to Ukraine’s default by the end of this spring,” said Alexander Paraschiy, the head of research at Concorde Capital investment bank in Kyiv. Most of the key players have offered their solution, except for the Yanukovych administration, which is still deciding. The proposals have turned out to be just as divergent as the inter- ests of the players involved, indicating that whatever decision is made will draw a fierce reaction from someone else. -

The Cases of Ła ´Ncut and Włodawa
arts Article The Question of Appropriateness. Museums Established in Synagogues in Communist Poland: The Cases of Ła ´ncutand Włodawa Kinga Migalska Prints and Drawings Cabinet, National Museum in Kraków, 3 May Street, 30-062 Kraków, Poland; [email protected] Received: 9 November 2019; Accepted: 12 December 2019; Published: 17 December 2019 Abstract: World War II and the subsequent period of communist rule severely diminished the amount of historic Jewish architecture in Poland. It is estimated that in the mid-1990s there were about 321 synagogues and prayer houses in the country, all in various states of preservation. This article examines two case studies of synagogues that were salvaged by being transformed into Judaica museums. The first of these is the synagogue in Ła´ncutand the second concerns the complex of two synagogues and one prayer house in Włodawa. The article contains an analysis of both examples from the perspective of the following factors: the circumstances under which the institution was established, the place that the history and culture of Jews took in the Museum’s activity, the way that Judaica collections and exhibitions were constructed, the substantive, educational, and research activities that were undertaken, as well as the issue of what place these monuments occupy in the town’s landscape. Keywords: synagogues; Jewish heritage; Judaica 1. Introduction From the second half of the 1980s, a growing interest in the heritage of Polish Jews was noticeable both within the country and abroad. This phenomenon developed in the 1990s, and especially intensified after 2000 (Poland’s accession to the European Union in 2004 was a watershed in this regard). -

Men's Basketball
Men’s Basketball Media Guide Men’s Basketball Media Guide 03 Content Venues’ information 4 Qualifying Process for London 2012 Olympic Basketball Tournament for Men 6 Competition system 7 Schedule Men 8 GROUP A 9 Argentina 10 France 14 Lithuania 18 Nigeria 22 Tunisia 26 USA 30 GROUP B 35 Australia 36 Brazil 40 China 44 Great Britain 48 Russia 52 Spain 56 Officials 60 HISTORY 61 2011 FIBA Africa Championship for Men 61 2011 FIBA Americas Championship for Men 62 2011 FIBA Asia Championship for Men 63 EuroBasket 2011 64 2011 FIBA Oceania Championship for Men 2012 FIBA Olympic Qualifying Tournament 65 Head-to-Head 66 FIBA Events History 70 Men Olympic History 72 FIBA Ranking 74 Competition schedule Women and Men 76 If you have any questions, please contact FIBA Communications at [email protected] Publisher: FIBA Production: Norac Presse Conception & editors: Pierre-Olivier MATIGOT (BasketNews) and FIBA Communications. Designer & Layout: Thierry DESCHAMPS (Zone Presse). Copyright FIBA 2012. The reproduction and photocopying, even of extracts, or the use of articles for commercial purposes without written prior approval by FIBA is prohibited. FIBA – Fédération Internationale de Basketball - Av. Louis Casaï 53 1216 Cointrin / Geneva - Switzerland 2012 MEN’S OLYMPICS 04 LONDON, 29 JULY - 12 AUGUST Venues’ information Olympic Basketball Arena Location: North side of the Olympic Park, Stratford Year of construction: 2010-2011 Spectators: 12,000 Estimated cost: £40,000,000 One of the largest temporary sporting venues ever built, the London Olympic Basketball Arena seats 12,000 spectators and will host all basketball games from the men’s and women’s tournaments except for the Quarter-Finals of the men’s event and the Semi-finals and Finals (men and women), which will be held at the North Greenwich Arena. -

RESEARCH REPORT NIFS-DATA Series
JP0355031 Bibliography of Electron and Photon Cross Sections with Atoms and Molecules Published in the 20'hCentury - Argon - M. Hayashi (Received Jan. 17, 2003 NIFS-DATA-72 Jan. 2003 41 410 01 RESEARCH REPORT NIFS-DATA Series This report was prepared as a preprint of work perfon-ned as a collaboration research of the National Institute for Fusion Science (NIFS) of Japan. The views presented here are solely those of the authors. Ibis document is intended for iformation only and may be published in a journal after some rearrangement of its contents in the future. Inquiries about copyright should be addressed to the Research Information Center, National Insitute for Fusion Science, Oroshi-cho, Toki-shi, Gifu-ken 509-5292 Japan. E-mail: bunkenCa)nifs.acjp <Notice about photocopying> In order to photocopy any work from this publication, you or your organization must obtain permission from the following organizaion which has been delegated for copyright for clearance by the copyright owner of this publication. Except in the USA Japan Academic Association for Copyright Clearance (JAACC) 41-6 Akasaka 9-chome, Minato-ku, Tokyo 107-0052 Japan TEL:81-3-3475-5618 FAX:81-3-3475-5619 E-mail:naka-atsumuj.biglobe.nejp In the USA Copyright Clearance Center, Inc. 222 Rosewood Drive, Danvers, MA 01923 USA Phone: 978) 750-8400 FAX: 978) 750-4744 Bibliography of Electron and Photon Cross Sections with Atoms and Molecules Published in the 20thCentury - Argon * Makoto Hayashi (Gaseous Electronics Institute) A bibliography of original and review reports of experiments or theories of eectron and photon cross sections and also electron swarm data are presented for atomic or molecular species with specified targets.