Seasonal Distribution of Sage-Grouse in Hamlin Valley, Utah and the Effect of Fences on Grouse and Avian Predators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
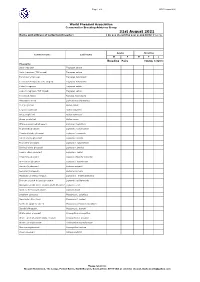
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor -

Complete Mitochondrial Genome of the Western Capercaillie Tetrao Urogallus (Phasianidae, Tetraoninae)
Zootaxa 4550 (4): 585–593 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4550.4.9 http://zoobank.org/urn:lsid:zoobank.org:pub:12E18262-0DCA-403A-B047-82CFE5E20373 Complete mitochondrial genome of the Western Capercaillie Tetrao urogallus (Phasianidae, Tetraoninae) GAËL ALEIX-MATA1,5, FRANCISCO J. RUIZ-RUANO2, JESÚS M. PÉREZ1, MATHIEU SARASA3 & ANTONIO SÁNCHEZ4 1Department of Animal and Plant Biology and Ecology, Jaén University, Campus Las Lagunillas, E-23071, Jaén, Spain. E-mail: [email protected] 2Departamento de Genética, Facultad de Ciencias, Universidad de Granada, Avda. Fuentenueva, 18071 Granada, Spain. 3BEOPS, 1 Esplanade Compans Caffarelli, 31000 Toulouse, France 4Department of Experimental Biology, Jaén University, Campus Las Lagunillas, E-23071, Jaén, Spain 5Corresponding author Gaël Aleix-Mata: [email protected] ORCID: 0000-0002-7429-4051 Francisco J. Ruíz-Ruano: [email protected] ORCID: 0000-0002-5391-301X Jesús M. Pérez: [email protected] ORCID: 0000-0001-9159-0365 Mathieu Sarasa: [email protected] ORDCID: 0000-0001-9067-7522 Antonio Sánchez: [email protected] ORCID: 0000-0002-6715-8158 Abstract The Western Capercaillie (Tetrao urogallus) is a galliform bird of boreal climax forests from Scandinavia to eastern Sibe- ria, with a fragmented population in southwestern Europe. We extracted the DNA of T. urogallus aquitanicus and obtained the complete mitochondrial genome (mitogenome) sequence by combining Illumina and Sanger sequencing sequence da- ta. The mitochondrial genome of T. urogallus is 16,683 bp long and is very similar to that of Lyrurus tetrix (16,677 bp). -

Western Capercaillie
WESTERN CAPERCAILLIE The grouse of the Today, it is an endangered species at Cansiglio namely due to the effects of the following factors: Cansiglio Forest • habitat loss, degradation and fragmentation due to lack of undergrowth, increase of natural A representative species of mountain and predators and climate changes; boreal ecosystems, the western capercaillie (Tetrao urogallus) is one of the species of grouse • diminishing undergrowth habitat resulting from inhabiting the Cansiglio Forest. deer grazing; • anthropic disturbances related to recreational Best known for the sensational displays of the activities. males during courtship, the western capercaillie lives in mature forests with large open areas and rich, yet not too thick, undergrowth, which Western capercaillie in the Cansiglio beechwood provides food, shelter and a place to nest in. (Photo: A. Ferro) In the Cansiglio Forest and other Rete Natura 2000 areas in Veneto, the periods in which tree cutting activities are allowed are compatible with this grouse’s breeding and rearing activities. Its presence is an assurance of quality for the environment, which is why improving its habitat means contributing to the increase of an area’s biodiversity. Western capercaillie (Photo: M. Bottazzo) Ideal habitat of the Western Capercaillie (Photo: R. Luise) Promuoviamo la Gestione Sostenibile delle Foreste PEFC/18-22-13/03 www.pefc.it Bio∆4 - “New instruments to enhance the biodiversity of cross-border forest ecosystems” ITAT2021 A project financed by the European Regional Development Fund (ERDF) within the context of the 2014 - 2020 Interreg V-A Italy - Austria programme (2017 call for tenders). The Italian portion is co-financed by the national rotating fund (C.I.P.E. -

Slovenská Poľnohospodárska Univerzita V Nitre
SLOVENSKÁ POĽNOHOSPODÁRSKA UNIVERZITA V NITRE FAKULTA AGROBIOLÓGIE A POTRAVINOVÝCH ZDROJOV 1131583 BIOLOGICKÁ A EKOLOGICKÁ CHARAKTERISTIKA DRUHOV ČEĽADE PHASIANIDAE 2011 Jozef Čepišák SLOVENSKÁ POĽNOHOSPODÁRSKA UNIVERZITA V NITRE FAKULTA AGROBIOLÓGIE A POTRAVINOVÝCH ZDROJOV BIOLOGICKÁ A EKOLOGICKÁ CHARAKTERISTIKA DRUHOV PODČEĽADE PHASIANIDAE (Bakalárska práca) Študijný program: Udržateľné poľnohospodárstvo a rozvoj vidieka Študijný odbor: 4140700 Všeobecné poľnohospodárstvo Školiace pracovisko: Katedra environmentalistiky a zoológie Školiteľ: RNDr. Alena Rakovská, CSc. Nitra 2011 Jozef Čepišák ČESTNÉ VYHLÁSENIE Podpísaný, Jozef Čepišák vyhlasujem, že som záverečnú prácu na tému „Biologická a ekologická charakteristika druhov čeľade Phasianidae“ vypracoval samostatne s použitím uvedenej literatúry. Som si vedomý zákonných dôsledkov v prípade, ak hore uvedené údaje nie sú pravdivé. V Nitre 12.5.2011 ........................ podpis 3 Poďakovanie Touto cestou si dovoľujem poďakovať vedúcej bakalárskej práce RNDr. Alene Rakovskej, CSc. za jej pomoc, cenné rady a pripomienky pri spracovaní bakalárskej práce. 4 ABSTRAKT V predloženej bakalárskej práci „Biologická a ekologická charakteristika druhov čeľade Phasianidae“ je uvedená morfologická a anatomická stavba tela vtákov, spoločné znaky triedy vtáky (Aves) a ich fylogenetický pôvod. V práci uvádzame taxonómiu vybraných druhov bažantov, ktorá je podľa rôznych autorov rozdielna. Rad hrabavce (Galliformes) je bohatý na druhy, s charakteristickými vlastnosťami, ktoré sme bližšie špecifikovali. Stručne uvádzame i význam týchto vtákov pre ľudstvo a ich vzťah k človeku. Najväčšie druhové zastúpenie v rade hrabavcov má čeľaď bažantovité (Phasianidae). Do tejto čeľade patria hospodársky významne druhy hydiny, chované hlavne na mäsovú produkciu a vajcia. Patria sem aj naše pôvodné druhy vtákov (tetrov hlucháň, jarabica poľná, jariabok hôrny) a jeden nepôvodný druh, ktorým je bažant poľovný (Phasianus colchicus), súhrnne nazývané pernatá zver, ktorá je významná z poľovníckeho hľadiska. -

Reconstructing the Diet of an Elusive Wood Grouse (Western Capercaillies) Using
RECONSTRUCTING THE DIET OF AN ELUSIVE WOOD GROUSE (WESTERN CAPERCAILLIES) USING METAGENOMICS displaying its magnificent tail feathers male capercaillie A Spotted! Per Gätzschmann CREDIT: PHOTO by PHYSILIA CHUA Department of Biology, University of Copenhagen, Copenhagen, Denmark Environmental DNA provides a non-invasive and simple means of biomonitoring one are the days when researchers needed to samples circumvents some of the challenges of study- spend countless hours observing an animal in ing animals otherwise hard to find. Gthe wild to understand its behaviour and ecol- One such NGS approach is metagenomics shotgun ogy. As we demonstrate with our study, valuable data sequencing (MSS), which determines the nucleotide can be gathered by simply examining faecal samples composition of large amounts of random DNA mole- with powerful metagenomics approaches. cules recovered from complex samples of DNA from The need for data that effectively informs biological various sources. This method makes it possible to conservation is intensifying as the rate of biodiversity simultaneously retrieve information about the host’s loss increases. Traditionally, scientists have endured diet, microbiome, gut parasites, as well as the popula- long hours in the field, often hiding uncomfortably tion structure of the species1. While it has vast poten- in bushes or traversing dangerous and hard-to-reach tial for conservation biology, few studies have utilised places, all for the purpose of observing elusive ani- MSS to reconstruct the diet of animals, and none have mals. done so for herbivorous birds. With the advent of next-generation sequencing The western capercaillie (Tetrao urogallus), or (NGS) technologies – those that effectively provide wood grouse, is an emblematic species which can large amounts of DNA sequence data – it is now pos- be found in the coniferous forest of Eurasia. -

Phasianinae Species Tree
Phasianinae Blood Pheasant,Ithaginis cruentus Ithaginini ?Western Tragopan, Tragopan melanocephalus Satyr Tragopan, Tragopan satyra Blyth’s Tragopan, Tragopan blythii Temminck’s Tragopan, Tragopan temminckii Cabot’s Tragopan, Tragopan caboti Lophophorini ?Snow Partridge, Lerwa lerwa Verreaux’s Monal-Partridge, Tetraophasis obscurus Szechenyi’s Monal-Partridge, Tetraophasis szechenyii Chinese Monal, Lophophorus lhuysii Himalayan Monal, Lophophorus impejanus Sclater’s Monal, Lophophorus sclateri Koklass Pheasant, Pucrasia macrolopha Wild Turkey, Meleagris gallopavo Ocellated Turkey, Meleagris ocellata Tetraonini Ruffed Grouse, Bonasa umbellus Hazel Grouse, Tetrastes bonasia Chinese Grouse, Tetrastes sewerzowi Greater Sage-Grouse / Sage Grouse, Centrocercus urophasianus Gunnison Sage-Grouse / Gunnison Grouse, Centrocercus minimus Dusky Grouse, Dendragapus obscurus Sooty Grouse, Dendragapus fuliginosus Sharp-tailed Grouse, Tympanuchus phasianellus Greater Prairie-Chicken, Tympanuchus cupido Lesser Prairie-Chicken, Tympanuchus pallidicinctus White-tailed Ptarmigan, Lagopus leucura Rock Ptarmigan, Lagopus muta Willow Ptarmigan / Red Grouse, Lagopus lagopus Siberian Grouse, Falcipennis falcipennis Spruce Grouse, Canachites canadensis Western Capercaillie, Tetrao urogallus Black-billed Capercaillie, Tetrao parvirostris Black Grouse, Lyrurus tetrix Caucasian Grouse, Lyrurus mlokosiewiczi Tibetan Partridge, Perdix hodgsoniae Gray Partridge, Perdix perdix Daurian Partridge, Perdix dauurica Reeves’s Pheasant, Syrmaticus reevesii Copper Pheasant, Syrmaticus -

Birds in the Ancient World from a to Z
BIRDS IN THE ANCIENT WORLD FROM A TO Z Why did Aristotle claim that male Herons’ eyes bleed during mating? Do Cranes winter near the source of the Nile? Was Lesbia’s pet really a House Sparrow? Ornithology was born in ancient Greece, when Aristotle and other writers studied and sought to identify birds. Birds in the Ancient World from A to Z gathers together the information available from classical sources, listing all the names that ancient Greeks gave their birds and all their descriptions and analyses. Arnott identifies (where achievable) as many of them as possible in the light of modern ornithological studies. The ancient Greek bird names are transliterated into English script, and all that the classical writers said about birds is presented in English. This book is accordingly the first complete discussion of classical bird names that will be accessible to readers without ancient Greek. The only previous study in English on the same scale was published over seventy years ago and required a knowledge of Greek and Latin. Since then there has been an enormous expansion in ornithological studies which has vastly increased our knowledge of birds, enabling us to evaluate (and explain) ancient Greek writings about birds with more confidence. With an exhaustive bibliography (partly classical scholarship and partly ornithological) added to encourage further study Birds in the Ancient World from A to Z is the definitive study of birds in the Greek and Roman world. W.Geoffrey Arnott is former Professor of Greek at the University of Leeds and Fellow of the British Academy. -
Capercaillie Conservation in Europe
Kandidatarbeten 2019:20 i skogsvetenskap Fakulteten för skogsvetenskap Limiting factors in capercaillie (Tetrao urogallus) conservation Tjäderförvaltningens begränsande faktorer Ferdinand Von Wright "Capercaillies Courting" 1864 in Ateneum Art museum, Helsinki . Christer Moreira Boman & Andreas Pettersson Sveriges Lantbruksuniversitet Självständigt arbete i skogsvetenskap, 15 hp Jägmästarprogrammet Umeå Kandidatarbeten i Skogsvetenskap Fakulteten för skogsvetenskap, Sveriges lantbruksuniversitet Institutionen för skogens ekologi och skötsel Enhet/Unit Department of Forest Ecology and Management Författare/Author Andreas Pettersson, Christer Moreira Boman Begränsande faktorer vid förvaltning av tjäder (Tetrao Titel, Sv urogallus) Titel, Eng Limiting factors in capercaillie (Tetrao urogallus) conservation Återintroduktion, tjäder, bevarande ekologi/ Reintroduction, Nyckelord/ capercaillie conservation, wildlife management, population Keywords viability analysis. John P. Ball ,Institutionen för vilt, fisk och miljö/ Department of Handledare/Supervisor Wildlife, Fish, and Environmental Studies Examinator/Examiner Tommy Mörling Institutionen för skogens ekologi och skötsel/ Department of Forest Ecology and Management Kurstitel/Course Kandidatarbete i skogsvetenskap Bachelor Degree in Forest Science Kurskod EX0911 Program Jägmästarprogrammet Omfattning på arbetet/ 15 hp Nivå och fördjupning på arbetet G2E Utgivningsort Umeå Utgivningsår 2019 Serie Kandidatarbeten i Skogsvetenskap Acknowledgements We want to thank our supervisor, John Ball for his time and guidance with this paper for our bachelor’s degree. His dedication and knowledge were greatly appreciated as it facilitated our work. ABSTRACT With current extinction rates comparable with the rates of previous mass extinctions and humanities necessity of biodiversity, conservation of habitat and species should be regarded as paramount whenever planning land use. In this paper we have focused on the Western capercaillie (Tetrao urogallus) an umbrella species in Eurasian boreal and temperate forests. -

Birdwatching Breaks 2021 Over 30 Years of Guided Tours Destinations
Birdwatching Breaks 2021 Over 30 years of guided tours Destinations BLACK ISLE BIRDING – SCOTLAND Scotland . Caithness and Orkney . 12 Scotland . Dumfries and Galloway with Northumberland . 14 Scotland . Scottish Highlands and Aberdeenshire . 16 Scotland . Isle of Islay . 18 Scotland . Mull, Tiree and the Uists . 20 Scotland . Scottish Highlands – spring . 25 Scotland . Scottish Highlands – autumn . 23 Scotland . Shetland – autumn . 28 Scotland . Shetland – breeding birds . 30 Scotland . Western Isles – the Uists, Harris and Lewis . 32 AFRICA Ethiopia . Birds and Mammals of north-east Africa . 34 Guinea-Bissau . Casamance and Guinea-Bissau . 38 Namibia . Birds of Etosha and the Skeleton Coast . 41 Rwanda . Albertine Rift endemics and mammals . 44 Senegal . SE Senegal and Saloum . 46 Senegal . Birds of the Sahel and Saloum Delta . .49 Senegal . Pelagic birding and birds and the Sahel . 53 ASIA AND AUSTRALASIA Japan . Breeding birds and migrants . 56 Japan . Winter birds in the Land of the Rising Sun . 59 Siberia . Autumn migration at Lake Baikal and Buryat . 62 EUROPE Bulgaria . Winter birds of the Black Sea . 66 England . West Cornwall . 68 England . Kent and Sussex in springtime . 71 France . Champagne-Ardennes at New Year 2021 . 74 France . Camargue and Corsica . 76 Ireland . Northern Ireland and Donegal . 78 Mallorca . Autumn in the Balearic Islands . 80 Norway . Winter birds of the High Arctic . 82 Sweden . Autumn migration at Falsterbo . 84 Sweden . Lake Hornborga . 86 THE AMERICAS Canada . Long Point, Algonquin and Carden Alvar . 88 Colombia . The world’s best birding country . 92 Mexico . Veracruz, Oaxaca and Sierra Madre . 96 Tr inidad and Grenada . Birds of the southern Caribbean . 100 Front cover: Western Capercaillie, taken near Lake Hornborga, Sweden. -

Outdoor Recreation Causes Effective Habitat Reduction in Capercaillie Tetrao Urogallus: a Major Threat for Geographically Restricted Populations
Journal of Avian Biology 48: 1583–1594, 2017 doi: 10.1111/jav.01239 © 2017 The Authors. Journal of Avian Biology © 2017 Nordic Society Oikos Subject Editor: Jan Engler. Editor-in-Chief: Jan-Åke Nilsson. Accepted 10 January 2017 Outdoor recreation causes effective habitat reduction in capercaillie Tetrao urogallus: a major threat for geographically restricted populations Joy Coppes, Judith Ehrlacher, Dominik Thiel, Rudi Suchant and Veronika Braunisch J. Coppes ([email protected]), J. Ehrlacher, R. Suchant and V. Braunisch, Forest Research Inst. of Baden-Wuerttemberg FVA, Freiburg, Germany. VB also at: Conservation Biology, Inst. of Ecology and Evolution, Univ. of Bern, Switzerland. – D. Thiel, Swiss Ornithological Inst., Sempach, Switzerland and Amt für Natur, Jagd und Fischerei, St Gallen, Switzerland. Outdoor recreation inflicts a wide array of impacts on individual animals, many of them reflected in the avoidance of disturbed areas. The scale and spatial extent, however, at which wildlife populations are affected, are mostly unclear. Particularly in geographically isolated populations, where restricted habitat availability may preclude a relocation to undisturbed areas, effective habitat reduction may remain underestimated or even unnoticed, when animals stay in disturbed areas and only show small-scale responses. Based on telemetry data, we investigated the spatial and seasonal effects of outdoor recreation – in relation to landscape and vegetation conditions – on western capercaillieTetrao urogallus, considering two scales, -

WPA Survey of Captive Birds 2020
Private Breeders & Zoo Collections (109) Approx 50% ringed Common name Latin name Adults Juveniles M F M F ? Breeding 12 Pairs 2020 Young Pairs Mth+ Satyr tragopan Tragopan satyra 31 32 14 19 5 Temminck's tragopan Tragopan temminckii 42 45 15 18 36 Cabot's tragopan Tragopan caboti 39 31 11 13 9 Koklass pheasant Pucrasia macrolopha 13 14 23 16 23 Himalayan monal Lophophorus impeyanus 23 25 11 7 36 Red junglefowl Gallus gallus 6 6 11 7 5 Ceylon junglefowl Gallus lafayettei 6 5 6 8 Grey junglefowl Gallus sonneratii 16 25 15 13 17 Green junglefowl Gallus varius 1 kalij pheasant - unknown subspecies Lophura leucomelana 3 1 1 White-crested kalij pheasant Lophura l. hamiltoni 5 4 2 Nepal Kalij pheasant Lophura l. leucomelana 7 7 1 4 Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata 1 1 2 1 Silver pheasant - unknown subspecies Lophura nycthemera 2 3 1 True silver pheasant Lophura n. nycthemera 15 18 26 23 5 Berlioz’s silver pheasant Lophura n. berliozi 2 2 Lewis’s silver pheasant Lophura n. lewisi 5 5 3 3 Imperial pheasant Lophura imperialis 1 1 Edwards's pheasant Lophura edwardsi edwardsi 72 66 21 22 18 Vietnamese pheasant Lophura e. hatinhensis 50 45 17 20 18 Swinhoe's pheasant Lophura swinhoii 11 13 4 4 6 Salvadori's pheasant Lophura inornata 6 4 1 Malaysian crestless fireback Lophura e. erythrophthalma 7 10 4 2 2 Bornean crested fireback pheasant Lophura i. ignita/nobilis 3 4 1 1 Malaysian crestless fireback/Vieillot's Lophura i. -

Galliforme Guidelines 260409
Guidelines for the Re-introduction of Galliformes for Conservation Purposes Edited by the World Pheasant Association and IUCN/SSC Re-introduction Specialist Group Occasional Paper of the IUCN Species Survival Commission No. 41 IUCN Founded in 1948, IUCN (International Union for the Conservation of Nature) brings together States, government agencies and a diverse range of nongovernmental organizations in a unique world partnership: over 1000 members in all, spread across some 160 countries. As a Union, IUCN seeks to influence, encourage and assist societies throughout the world to conserve the integrity and diversity of nature and to ensure that any use of natural resources is equitable and ecologically sustainable. IUCN builds on the strengths of its members, networks and partners to enhance their capacity and to support global alliances to safeguard natural resources at local, regional and global levels. IUCN Species Survival Commission (SSC) The IUCN Species Survival Commission is a science-based network of close to 8,000 volunteer experts from almost every country of the world, all working together towards achieving the vision of, “A world that values and conserves present levels of biodiversity.” WPA-IUCN SSC Galliformes Specialist Groups The Grouse Specialist Group was established in 1993 when it developed from WPA’s long- standing grouse network. It was designed to be a global voluntary network of individuals involved in the study, conservation, and sustainable management of grouse. The Pheasant Specialist Group was established in 1991 as a voluntary self-help network of scientists, wildlife managers, conservationists, aviculturists and educators. It was particularly concerned with the plight of threatened pheasant species, and ensuring the survival of viable populations in their natural habitats whilst enhancing the quality of human life.