Vocal Specialization Through Tracheal Elongation in an Extinct Miocene Pheasant from China Received: 7 November 2017 Zhiheng Li1,2, Julia A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nogth AMERICAN BIRDS
CHECK-LIST OF NOgTH AMERICAN BIRDS The Speciesof Birds of North America from the Arctic through Panama, Including the West Indies and Hawaiian Islands PREPARED BY THE COMMITTEE ON CLASSIFICATION AND NOMENCLATURE OF THE AMERICAN ORNITHOLOGISTS' UNION SEVENTH EDITION 1998 Zo61ogical nomenclature is a means, not an end, to Zo61ogical Science PUBLISHED BY THE AMERICAN ORNITHOLOGISTS' UNION 1998 Copyright 1998 by The American Ornithologists' Union All rights reserved, except that pages or sections may be quoted for research purposes. ISBN Number: 1-891276-00-X Preferred citation: American Ornithologists' Union. 1983. Check-list of North American Birds. 7th edition. American Ornithologists' Union, Washington, D.C. Printed by Allen Press, Inc. Lawrence, Kansas, U.S.A. CONTENTS DEDICATION ...................................................... viii PREFACE ......................................................... ix LIST OF SPECIES ................................................... xvii THE CHECK-LIST ................................................... 1 I. Tinamiformes ............................................. 1 1. Tinamidae: Tinamous .................................. 1 II. Gaviiformes .............................................. 3 1. Gaviidae: Loons ....................................... 3 III. Podicipediformes.......................................... 5 1. Podicipedidae:Grebes .................................. 5 IV. Procellariiformes .......................................... 9 1. Diomedeidae: Albatrosses ............................. -

Wild Turkey Education Guide
Table of Contents Section 1: Eastern Wild Turkey Ecology 1. Eastern Wild Turkey Quick Facts………………………………………………...pg 2 2. Eastern Wild Turkey Fact Sheet………………………………………………….pg 4 3. Wild Turkey Lifecycle……………………………………………………………..pg 8 4. Eastern Wild Turkey Adaptations ………………………………………………pg 9 Section 2: Eastern Wild Turkey Management 1. Wild Turkey Management Timeline…………………….……………………….pg 18 2. History of Wild Turkey Management …………………...…..…………………..pg 19 3. Modern Wild Turkey Management in Maryland………...……………………..pg 22 4. Managing Wild Turkeys Today ……………………………………………….....pg 25 Section 3: Activity Lesson Plans 1. Activity: Growing Up WILD: Tasty Turkeys (Grades K-2)……………..….…..pg 33 2. Activity: Calling All Turkeys (Grades K-5)………………………………..…….pg 37 3. Activity: Fit for a Turkey (Grades 3-5)…………………………………………...pg 40 4. Activity: Project WILD adaptation: Too Many Turkeys (Grades K-5)…..…….pg 43 5. Activity: Project WILD: Quick, Frozen Critters (Grades 5-8).……………….…pg 47 6. Activity: Project WILD: Turkey Trouble (Grades 9-12………………….……....pg 51 7. Activity: Project WILD: Let’s Talk Turkey (Grades 9-12)..……………..………pg 58 Section 4: Additional Activities: 1. Wild Turkey Ecology Word Find………………………………………….…….pg 66 2. Wild Turkey Management Word Find………………………………………….pg 68 3. Turkey Coloring Sheet ..………………………………………………………….pg 70 4. Turkey Coloring Sheet ..………………………………………………………….pg 71 5. Turkey Color-by-Letter……………………………………..…………………….pg 72 6. Five Little Turkeys Song Sheet……. ………………………………………….…pg 73 7. Thankful Turkey…………………..…………………………………………….....pg 74 8. Graph-a-Turkey………………………………….…………………………….…..pg 75 9. Turkey Trouble Maze…………………………………………………………..….pg 76 10. What Animals Made These Tracks………………………………………….……pg 78 11. Drinking Straw Turkey Call Craft……………………………………….….……pg 80 Section 5: Wild Turkey PowerPoint Slide Notes The facilities and services of the Maryland Department of Natural Resources are available to all without regard to race, color, religion, sex, sexual orientation, age, national origin or physical or mental disability. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

A Baraminological Analysis of the Land Fowl (Class Aves, Order Galliformes)
Galliform Baraminology 1 Running Head: GALLIFORM BARAMINOLOGY A Baraminological Analysis of the Land Fowl (Class Aves, Order Galliformes) Michelle McConnachie A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2007 Galliform Baraminology 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ Timothy R. Brophy, Ph.D. Chairman of Thesis ______________________________ Marcus R. Ross, Ph.D. Committee Member ______________________________ Harvey D. Hartman, Th.D. Committee Member ______________________________ Judy R. Sandlin, Ph.D. Assistant Honors Program Director ______________________________ Date Galliform Baraminology 3 Acknowledgements I would like to thank my Lord and Savior, Jesus Christ, without Whom I would not have had the opportunity of being at this institution or producing this thesis. I would also like to thank my entire committee including Dr. Timothy Brophy, Dr. Marcus Ross, Dr. Harvey Hartman, and Dr. Judy Sandlin. I would especially like to thank Dr. Brophy who patiently guided me through the entire research and writing process and put in many hours working with me on this thesis. Finally, I would like to thank my family for their interest in this project and Robby Mullis for his constant encouragement. Galliform Baraminology 4 Abstract This study investigates the number of galliform bird holobaramins. Criteria used to determine the members of any given holobaramin included a biblical word analysis, statistical baraminology, and hybridization. The biblical search yielded limited biosystematic information; however, since it is a necessary and useful part of baraminology research it is both included and discussed. -

Accurate Characterization of the IFITM Locus Using Miseq and Pacbio Sequencing Shows Genetic Variation in Galliformes
Bassano et al. BMC Genomics (2017) 18:419 DOI 10.1186/s12864-017-3801-8 RESEARCH ARTICLE Open Access Accurate characterization of the IFITM locus using MiSeq and PacBio sequencing shows genetic variation in Galliformes Irene Bassano1,2, Swee Hoe Ong1, Nathan Lawless3, Thomas Whitehead3, Mark Fife3 and Paul Kellam1,2* Abstract Background: Interferon inducible transmembrane (IFITM) proteins are effectors of the immune system widely characterized for their role in restricting infection by diverse enveloped and non-enveloped viruses. The chicken IFITM (chIFITM)genesareclusteredonchromosome5andtodate four genes have been annotated, namely chIFITM1, chIFITM3, chIFITM5 and chIFITM10. However, due to poor assembly of this locus in the Gallus Gallus v4 genome, accurate characterization has so far proven problematic. Recently, a new chicken reference genome assembly Gallus Gallus v5 was generated using Sanger, 454, Illumina and PacBio sequencing technologies identifying considerable differences in the chIFITM locus over the previous genome releases. Methods: We re-sequenced the locus using both Illumina MiSeq and PacBio RS II sequencing technologies and we mapped RNA-seq data from the European Nucleotide Archive (ENA) to this finalized chIFITM locus. Using SureSelect probes capture probes designed to the finalized chIFITM locus, we sequenced the locus of a different chicken breed, namely a White Leghorn, and a turkey. Results: We confirmed the Gallus Gallus v5 consensus except for two insertions of 5 and 1 base pair within the chIFITM3 and B4GALNT4 genes, respectively, and a single base pair deletion within the B4GALNT4 gene. The pull down revealed a singleaminoacidsubstitutionofA63VintheCILdomainofIFITM2comparedtoRedJunglefowland13,13and11 differences between IFITM1, 2 and 3 of chickens and turkeys, respectively. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Incubator Birds: Biogeographical Origins and Evolution Of
Journal of Biogeography (J. Biogeogr.) (2014) 41, 2045–2056 ORIGINAL Incubator birds: biogeographical origins ARTICLE and evolution of underground nesting in megapodes (Galliformes: Megapodiidae) Rebecca B. Harris1,2*, Sharon M. Birks2 and Adam D. Leache1,2 1Department of Biology, University of ABSTRACT Washington, Seattle, WA 98195, USA, 2Burke Aim Unique amongst birds, megapodes (family Megapodiidae) have exchanged Museum of Natural History and Culture, the strategy of incubating eggs with the warmth of their bodies for incubation University of Washington, Seattle, WA 98195, USA behaviours that rely entirely on environmental heat sources. Typically, mound- builders capture heat released from the decomposition of organic materials, while burrow-nesters lay their eggs in geothermal or solar-heated soils. The evolutionary path towards novel incubation behaviours has led to ecological and physiological adaptations unique to megapodes. Here, we present a species tree for all extant megapodes that settles long-standing debates about mega- pode evolution: namely, their biogeographical origins and ancestral nesting behaviour. Location Australasia. Methods A time-calibrated multilocus species tree for all extant megapodes was constructed using *beast. We estimated and compared divergence dates for megapodes obtained from molecular rates, fossils, and a combination of fossils and rates. Using this tree, Bayesian estimation of ancestral nesting behaviour was conducted in BayesTraits and ancestral ranges were estimated in BioGeoBEARS. Results Recent dispersal has led to the recolonization of mainland Australia and New Guinea by Megapodius. Bayesian estimation of ancestral states indi- cates that mound building is the most probable ancestral nesting behaviour in megapodes (posterior probability = 0.75). Burrow nesting was acquired early in the diversification of the family (at least 14 Ma), followed by a single switch back to mound building. -

Compendium of Avian Ecology
Compendium of Avian Ecology ZOL 360 Brian M. Napoletano All images taken from the USGS Patuxent Wildlife Research Center. http://www.mbr-pwrc.usgs.gov/id/framlst/infocenter.html Taxonomic information based on the A.O.U. Check List of North American Birds, 7th Edition, 1998. Ecological Information obtained from multiple sources, including The Sibley Guide to Birds, Stokes Field Guide to Birds. Nest and other images scanned from the ZOL 360 Coursepack. Neither the images nor the information herein be copied or reproduced for commercial purposes without the prior consent of the original copyright holders. Full Species Names Common Loon Wood Duck Gaviiformes Anseriformes Gaviidae Anatidae Gavia immer Anatinae Anatini Horned Grebe Aix sponsa Podicipediformes Mallard Podicipedidae Anseriformes Podiceps auritus Anatidae Double-crested Cormorant Anatinae Pelecaniformes Anatini Phalacrocoracidae Anas platyrhynchos Phalacrocorax auritus Blue-Winged Teal Anseriformes Tundra Swan Anatidae Anseriformes Anatinae Anserinae Anatini Cygnini Anas discors Cygnus columbianus Canvasback Anseriformes Snow Goose Anatidae Anseriformes Anatinae Anserinae Aythyini Anserini Aythya valisineria Chen caerulescens Common Goldeneye Canada Goose Anseriformes Anseriformes Anatidae Anserinae Anatinae Anserini Aythyini Branta canadensis Bucephala clangula Red-Breasted Merganser Caspian Tern Anseriformes Charadriiformes Anatidae Scolopaci Anatinae Laridae Aythyini Sterninae Mergus serrator Sterna caspia Hooded Merganser Anseriformes Black Tern Anatidae Charadriiformes Anatinae -
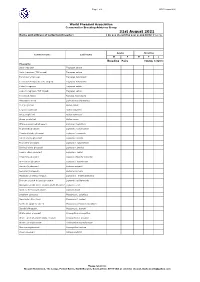
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor -

Complete Mitochondrial Genome of the Western Capercaillie Tetrao Urogallus (Phasianidae, Tetraoninae)
Zootaxa 4550 (4): 585–593 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4550.4.9 http://zoobank.org/urn:lsid:zoobank.org:pub:12E18262-0DCA-403A-B047-82CFE5E20373 Complete mitochondrial genome of the Western Capercaillie Tetrao urogallus (Phasianidae, Tetraoninae) GAËL ALEIX-MATA1,5, FRANCISCO J. RUIZ-RUANO2, JESÚS M. PÉREZ1, MATHIEU SARASA3 & ANTONIO SÁNCHEZ4 1Department of Animal and Plant Biology and Ecology, Jaén University, Campus Las Lagunillas, E-23071, Jaén, Spain. E-mail: [email protected] 2Departamento de Genética, Facultad de Ciencias, Universidad de Granada, Avda. Fuentenueva, 18071 Granada, Spain. 3BEOPS, 1 Esplanade Compans Caffarelli, 31000 Toulouse, France 4Department of Experimental Biology, Jaén University, Campus Las Lagunillas, E-23071, Jaén, Spain 5Corresponding author Gaël Aleix-Mata: [email protected] ORCID: 0000-0002-7429-4051 Francisco J. Ruíz-Ruano: [email protected] ORCID: 0000-0002-5391-301X Jesús M. Pérez: [email protected] ORCID: 0000-0001-9159-0365 Mathieu Sarasa: [email protected] ORDCID: 0000-0001-9067-7522 Antonio Sánchez: [email protected] ORCID: 0000-0002-6715-8158 Abstract The Western Capercaillie (Tetrao urogallus) is a galliform bird of boreal climax forests from Scandinavia to eastern Sibe- ria, with a fragmented population in southwestern Europe. We extracted the DNA of T. urogallus aquitanicus and obtained the complete mitochondrial genome (mitogenome) sequence by combining Illumina and Sanger sequencing sequence da- ta. The mitochondrial genome of T. urogallus is 16,683 bp long and is very similar to that of Lyrurus tetrix (16,677 bp). -

Western Capercaillie
WESTERN CAPERCAILLIE The grouse of the Today, it is an endangered species at Cansiglio namely due to the effects of the following factors: Cansiglio Forest • habitat loss, degradation and fragmentation due to lack of undergrowth, increase of natural A representative species of mountain and predators and climate changes; boreal ecosystems, the western capercaillie (Tetrao urogallus) is one of the species of grouse • diminishing undergrowth habitat resulting from inhabiting the Cansiglio Forest. deer grazing; • anthropic disturbances related to recreational Best known for the sensational displays of the activities. males during courtship, the western capercaillie lives in mature forests with large open areas and rich, yet not too thick, undergrowth, which Western capercaillie in the Cansiglio beechwood provides food, shelter and a place to nest in. (Photo: A. Ferro) In the Cansiglio Forest and other Rete Natura 2000 areas in Veneto, the periods in which tree cutting activities are allowed are compatible with this grouse’s breeding and rearing activities. Its presence is an assurance of quality for the environment, which is why improving its habitat means contributing to the increase of an area’s biodiversity. Western capercaillie (Photo: M. Bottazzo) Ideal habitat of the Western Capercaillie (Photo: R. Luise) Promuoviamo la Gestione Sostenibile delle Foreste PEFC/18-22-13/03 www.pefc.it Bio∆4 - “New instruments to enhance the biodiversity of cross-border forest ecosystems” ITAT2021 A project financed by the European Regional Development Fund (ERDF) within the context of the 2014 - 2020 Interreg V-A Italy - Austria programme (2017 call for tenders). The Italian portion is co-financed by the national rotating fund (C.I.P.E. -

Peacock-Pheasants and Asiatic Spurfowl
Above: Adult male Burmese Grey Chinquis Polyplectron b. bicalcaratum in its native habitat. Note multiple metatarsal kicking thorns. Photo P. Kittipinyowat. PEACOCK-PHEASANTS AND ASIATIC SPURFOWL By: Kermit Blackwood (USA) PART 1 INTRODUCTION TO PEACOCK-PHEASANTS In this article, we examine in detail several tropical Asian galliforms frequently mentioned in technical ornithological literature but rarely discussed at length, the peacock-pheasants, those members of the genus Polyplectron, conventionally considered *a as deep forest-adapted, evolutionary prototypes of the larger and even more elaborately plumaged argus and peafowl. A theory that’s become a matter of tradition places the peacock-pheasant as a phylogenetic link between pheasants and peafowl. In this article we will have an additional look at some obscure galliforms even more poorly known than peacock-pheasants, namely the mysterious, Crimson-headed partridge (Haematortyx sanguiniceps) endemic to the mountains of Borneo and the Indian Subcontinent’s enigmatic Asiatic spurfowl of the genus Galloperdix. These two genera share a number of unusual features with peacock-pheasants that may suggest a monophyletic origin. Nine peacock-pheasant species are presently recognised, all united within the genus Polyplectron. Two forms once identified as subspecies by some authors are currently regarded as distinct species. The Hainan peacock-pheasant P. katsumatae and the Bornean peacock-pheasant P. schleiermacheri were both treated as distinct species until Jean Delacour’s reclassification scheme circa 1977, when each were demoted to subspecific status; the Hainan as subspecific of the Grey and the Bornean as a subspecies of the Malayan. Biogeographical, molecular, morphological and phenotypic datas have re- established their species status.