A SUPERTREE of BIRDS Katie E. Davis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
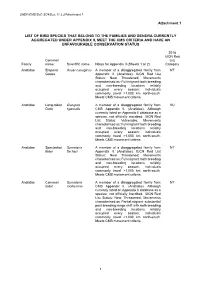
Attachment 1 LIST of BIRD SPECIES THAT BELONG to the FAMILIES
UNEP/CMS/ScC-SC4/Doc.11.3.2/Attachment 1 Attachment 1 LIST OF BIRD SPECIES THAT BELONG TO THE FAMILIES AND GENERA CURRENTLY AGGREGATED UNDER APPENDIX II, MEET THE CMS CRITERIA AND HAVE AN UNFAVOURABLE CONSERVATION STATUS 2018 IUCN Red Common List Family name Scientific name Notes for Appendix II (Sheets 1 or 2) Category Anatidae Emperor Anser canagicus A member of a disaggregated family from NT Goose Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Long-tailed Clangula A member of a disaggregated family from VU Duck hyemalis CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Vulnerable; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Spectacled Somateria A member of a disaggregated family from NT Eider fischeri Appendix II. (Anatidae). IUCN Red List Status: Near Threatened; Movements characterised as: Full migrant: both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. Meets CMS movement criteria. Anatidae Common Somateria A member of a disaggregated family from NT Eider mollissima CMS Appendix II. (Anatidae). Although currently listed on Appendix II database as a species, not officially inscribed. IUCN Red List Status: Near Threatened; Movements characterised as: Partial migrant: substantial post-breeding range shift with both breeding and non-breeding locations reliably occupied every season; individuals commonly travel >1,000 km north-south. -

Predicting the Distribution of the Vulnerable Yellow-Breasted Pipit (Anthus Chloris) Using Species Distribution Modelling
1 Predicting the distribution of the Vulnerable Yellow-breasted Pipit (Anthus chloris) using Species Distribution Modelling Darren W. Pietersen1*, Ian T. Little2, Raymond Jansen3 and Andrew E. McKechnie1 1DST-NRF Centre of Excellence at the Percy FitzPatrick Institute, Department of Zoology and Entomology, University of Pretoria, Private Bag X20, Hatfield, 0028, South Africa 2The Endangered Wildlife Trust, Private Bag X11, Modderfontein, 1645, Johannesburg, South Africa 3Department of Environmental, Water and Earth Sciences, Tshwane University of Technology, Private Bag X680, Pretoria, 0001, South Africa *Address for correspondence: Department of Zoology and Entomology University of Pretoria Private Bag X20 Hatfield 0028 [email protected] +27 82 937 6052 2 Abstract The Yellow-breasted Pipit (Anthus chloris) is endemic to the eastern escarpment of South Africa, marginally entering eastern Lesotho. This species is classified as globally Vulnerable due to a perceived decreasing population size and loss of habitat. We employed Species Distribution Modelling to investigate the predicted range of this species to determine whether additional purportedly suitable habitat exists where this species may be found, and to assess the degree to which habitat loss has affected this species. We used a database of 250 independently obtained and verified sightings to predict the summer breeding distribution of this species and compare our verified sightings and predicted range to the sightings currently in the Second Southern African Bird Atlas Project (SABAP2) database and the latest regional conservation assessment. Our models closely approximate the current distribution of the Yellow-breasted Pipit, and suggest that most of the purportedly suitable habitat is occupied, at least at the macro-scale. -

The Birds (Aves) of Oromia, Ethiopia – an Annotated Checklist
European Journal of Taxonomy 306: 1–69 ISSN 2118-9773 https://doi.org/10.5852/ejt.2017.306 www.europeanjournaloftaxonomy.eu 2017 · Gedeon K. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:A32EAE51-9051-458A-81DD-8EA921901CDC The birds (Aves) of Oromia, Ethiopia – an annotated checklist Kai GEDEON 1,*, Chemere ZEWDIE 2 & Till TÖPFER 3 1 Saxon Ornithologists’ Society, P.O. Box 1129, 09331 Hohenstein-Ernstthal, Germany. 2 Oromia Forest and Wildlife Enterprise, P.O. Box 1075, Debre Zeit, Ethiopia. 3 Zoological Research Museum Alexander Koenig, Centre for Taxonomy and Evolutionary Research, Adenauerallee 160, 53113 Bonn, Germany. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] 1 urn:lsid:zoobank.org:author:F46B3F50-41E2-4629-9951-778F69A5BBA2 2 urn:lsid:zoobank.org:author:F59FEDB3-627A-4D52-A6CB-4F26846C0FC5 3 urn:lsid:zoobank.org:author:A87BE9B4-8FC6-4E11-8DB4-BDBB3CFBBEAA Abstract. Oromia is the largest National Regional State of Ethiopia. Here we present the first comprehensive checklist of its birds. A total of 804 bird species has been recorded, 601 of them confirmed (443) or assumed (158) to be breeding birds. At least 561 are all-year residents (and 31 more potentially so), at least 73 are Afrotropical migrants and visitors (and 44 more potentially so), and 184 are Palaearctic migrants and visitors (and eight more potentially so). Three species are endemic to Oromia, 18 to Ethiopia and 43 to the Horn of Africa. 170 Oromia bird species are biome restricted: 57 to the Afrotropical Highlands biome, 95 to the Somali-Masai biome, and 18 to the Sudan-Guinea Savanna biome. -

Insular Vertebrate Evolution: the Palaeontological Approach': Monografies De La Societat D'història Natural De Les Balears, Lz: Z05-118
19 DESCRIPTION OF THE SKULL OF THE GENUS SYLVIORNIS POPLIN, 1980 (AVES, GALLIFORMES, SYLVIORNITHIDAE NEW FAMILY), A GIANT EXTINCT BIRD FROM THE HOLOCENE OF NEW CALEDONIA Cécile MOURER-CHAUVIRÉ & Jean Christophe BALOUET MOUREH-CHAUVIRÉ, C. & BALOUET, l.C, zoos. Description of the skull of the genus Syluiornis Poplin, 1980 (Aves, Galliformes, Sylviornithidae new family), a giant extinct bird from the Holocene of New Caledonia. In ALCOVER, J.A. & BaVER, P. (eds.): Proceedings of the International Symposium "Insular Vertebrate Evolution: the Palaeontological Approach': Monografies de la Societat d'Història Natural de les Balears, lZ: Z05-118. Resum El crani de Sylviornismostra una articulació craniarostral completament mòbil, amb dos còndils articulars situats sobre el rostrum, el qual s'insereix al crani en dues superfícies articulars allargades. La presència de dos procesos rostropterigoideus sobre el basisfenoide del rostrum i la forma dels palatins permet confirmar que aquest gènere pertany als Galliformes, però les característiques altament derivades del crani justifiquen el seu emplaçament a una nova família, extingida, Sylviornithidae. El crani de Syluiornis està extremadament eixamplat i dorsoventralment aplanat, mentre que el rostrum és massís, lateralment comprimit, dorsoventralment aixecat i mostra unes cristae tomiales molt fondes. El rostrum exhibeix un ornament ossi gran. La mandíbula mostra una símfisi molt allargada, les branques laterals també presenten unes cristae tomiales fondes, i la part posterior de la mandíbula és molt gruixada. Es discuteix el possible origen i l'alimentació de Syluiornis. Paraules clau: Aves, Galliformes, Extinció, Holocè, Nova Caledònia. Abstract The skull of Syluiornis shows a completely mobile craniorostral articulation, with two articular condyles situated on the rostrum, which insert into two elongated articular surfaces on the cranium. -

Ghana Mega Rockfowl & Upper Guinea Specials 3 to 25 January 2016 (23 Days) Trip Report
Knox Ghana Mega Rockfowl & Upper Guinea Specials 3 to 25 January 2016 (23 days) Trip Report Akun Eagle-Owl by David Hoddinott Trip Report compiled by Tour Leader Markus Lilje RBT Knox Ghana Mega Trip Report January 2015 2 Trip Summary Our private Ghana Mega trip proved yet again to be a resounding success! We notched up a fantastic species total in 23 days, where we covered the length and breadth of the country and a great variety of habitats in this superb West African country! Our tour started off with a visit to Shai Hills. This small but fabulous reserve has a nice variety of habitats including mixed woodland, grassland, wetlands and granite outcrops and therefore supports an interesting array of bird species. During our morning exploring the reserve we recorded African Cuckoo-Hawk, Western Marsh Harrier, Red-necked Buzzard, stunning Violet Turaco, numerous immaculate Blue-bellied Roller, Vieillot’s and Double-toothed Barbets, Senegal and African Wattled Lapwings, White-shouldered Black Tit, Red- shouldered Cuckooshrike, Black-bellied Bustard, Senegal Parrot, Senegal Batis and restless Senegal Eremomela. A number of migrants were seen including Willow Warbler, Whinchat and Spotted Flycatcher. Even mammals showed well for us as we had a number of Kob, Bushbuck, Olive Baboon, Callithrix Monkey and unusually good views of Lesser Spot- Blue-bellied Roller by Markus Lilje nosed Monkey! Well pleased with our morning’s birding, we left Shai Hills and made our way to Ho. En route we stopped for lunch near the Volta Dam where we enjoyed most memorable close-up encounters with Mangrove Sunbird and Bronze- tailed Starling. -

COSTA RICA: the Introtour (Group 1) Feb 2017
Tropical Birding Trip Report COSTA RICA: The Introtour (Group 1) Feb 2017 A Tropical Birding set departure tour COSTA RICA: The Introtour 13th - 23rd February 2017 (Group 1) Tour Leader: Sam Woods (Report and all photos by Sam Woods) This Keel-billed Toucan lit up our first afternoon, near Braulio Carrillo National Park. The same day also featured Thicket Antpitta and THREE species of owl during the daytime… Ferruginous Pygmy, Crested and Spectacled Owls. 1 www.tropicalbirding.com +1-409-515-9110 [email protected] Page Tropical Birding Trip Report COSTA RICA: The Introtour (Group 1) Feb 2017 INTRODUCTION There can be few countries in the World as welcoming to birders as Costa Rica; everywhere we went birds were plentiful and frequently people with binoculars were in attendance too. Indeed, Costa Rica makes you feel odd if you are NOT wearing a pair. We enjoyed a fantastic tour of some of the most revered sites in Costa Rican birding; we started out near San Jose in the dry Central Valley, before driving over to the Caribbean side, where foothill birding was done in and around Braulio Carrillo National Park, and held beautiful birds from the outset, like Black-and-yellow Tanager, Black-thighed Grosbeak, and daytime Spectacled and Crested Owls. A tour first was also provided by a Thicket Antpitta seen well by all. From there we continued downslope to the lowlands of that side, and the world famous La Selva Biological Station. La Selva is a place where birds feel particularly plentiful, and we racked up a heady list of birds on our one and a half days there, including Rufous and Broad-billed Motmots, Black-throated Trogon, Pale-billed, Cinnamon and Chestnut-colored Woodpeckers, Keel-billed and Yellow-throated Toucans, and Great Curassow, to name just a few of the highlights, which also included several two-toed sloths, the iconic Red-eyed Tree Frog (photo last page), and Strawberry Poison Dart Frogs of the much publicized “blue jeans” form that adorns so many tourist posters in this Sarapiqui region. -

A Baraminological Analysis of the Land Fowl (Class Aves, Order Galliformes)
Galliform Baraminology 1 Running Head: GALLIFORM BARAMINOLOGY A Baraminological Analysis of the Land Fowl (Class Aves, Order Galliformes) Michelle McConnachie A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2007 Galliform Baraminology 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ Timothy R. Brophy, Ph.D. Chairman of Thesis ______________________________ Marcus R. Ross, Ph.D. Committee Member ______________________________ Harvey D. Hartman, Th.D. Committee Member ______________________________ Judy R. Sandlin, Ph.D. Assistant Honors Program Director ______________________________ Date Galliform Baraminology 3 Acknowledgements I would like to thank my Lord and Savior, Jesus Christ, without Whom I would not have had the opportunity of being at this institution or producing this thesis. I would also like to thank my entire committee including Dr. Timothy Brophy, Dr. Marcus Ross, Dr. Harvey Hartman, and Dr. Judy Sandlin. I would especially like to thank Dr. Brophy who patiently guided me through the entire research and writing process and put in many hours working with me on this thesis. Finally, I would like to thank my family for their interest in this project and Robby Mullis for his constant encouragement. Galliform Baraminology 4 Abstract This study investigates the number of galliform bird holobaramins. Criteria used to determine the members of any given holobaramin included a biblical word analysis, statistical baraminology, and hybridization. The biblical search yielded limited biosystematic information; however, since it is a necessary and useful part of baraminology research it is both included and discussed. -

A Comprehensive Species-Level Molecular Phylogeny of the New World
YMPEV 4758 No. of Pages 19, Model 5G 2 December 2013 Molecular Phylogenetics and Evolution xxx (2013) xxx–xxx 1 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev 5 6 3 A comprehensive species-level molecular phylogeny of the New World 4 blackbirds (Icteridae) a,⇑ a a b c d 7 Q1 Alexis F.L.A. Powell , F. Keith Barker , Scott M. Lanyon , Kevin J. Burns , John Klicka , Irby J. Lovette 8 a Department of Ecology, Evolution and Behavior, and Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 9 55108, USA 10 b Department of Biology, San Diego State University, San Diego, CA 92182, USA 11 c Barrick Museum of Natural History, University of Nevada, Las Vegas, NV 89154, USA 12 d Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA 1314 15 article info abstract 3117 18 Article history: The New World blackbirds (Icteridae) are among the best known songbirds, serving as a model clade in 32 19 Received 5 June 2013 comparative studies of morphological, ecological, and behavioral trait evolution. Despite wide interest in 33 20 Revised 11 November 2013 the group, as yet no analysis of blackbird relationships has achieved comprehensive species-level sam- 34 21 Accepted 18 November 2013 pling or found robust support for most intergeneric relationships. Using mitochondrial gene sequences 35 22 Available online xxxx from all 108 currently recognized species and six additional distinct lineages, together with strategic 36 sampling of four nuclear loci and whole mitochondrial genomes, we were able to resolve most relation- 37 23 Keywords: ships with high confidence. -

State of Africa's Birds
An assessment by the BirdLife Africa Partnership1 State of Africa’s birds INTRODUCTION: The importance of birds and biodiversity Biodiversity Foreword underpins In 2009, BirdLife Botswana, the BirdLife Partner in Botswana, working with the Government of Botswana, established a Bird Population Monitoring (BPM) Programme. The BPM Programme is part of our lives the global Wild Bird Index effort, which uses information on birds to assess the overall condition of ecosystems and the environment on which we all depend. These trends will be used to set Africa is rich in its variety of conservation priorities, report on biodiversity changes (including the response of fauna and flora to living things, together referred climate change), as well as serve as useful inputs to State Of the Environment Reports and national to as biodiversity. Biodiversity reports to the Convention on Biological Diversity (CBD). is fundamental to human wellbeing: it offers multiple Currently there are over 350 volunteers supporting the programme who regularly monitor 241 transects spread throughout the country. My Government has been particularly supportive of the BPM opportunities for development Programme because it, among other things, bolsters the participation of rural communities in natural and improving livelihoods. resources management. Additionally, analysis of bird data will influence environmental policies and It is the basis for essential their implementation (e.g. game bird hunting quotas, and the control of the Red-billed Quelea), environmental services upon land-use planning and tourism development. The science of using bird information by the BirdLife which life on earth depends. Global Partnership to inform policies has far reaching impacts from local to global level. -

Ghana Nov 2013
Wise Birding Holidays All tours donate to conservation projects worldwide! Trip Report GHANA: Picathartes to Plover Tour Monday 18th Nov. - Tuesday 3rd Dec. 2013 Tour Participants: John Archer, Bob Watts, Graeme Spinks, Peter Alfrey, Nigel & Kath Oram Leaders: Chris Townend & Robert Oteng-Appau HIGHLIGHTS OF TRIP Yellow-headed Picathartes: Whilst sitting quietly in the magical forest, we were wowed by at least 5 different birds as they returned to their roost site. Egyptian Plover: A minimum of 5 adults were seen very well on the White Volta River, along with a very young chick. Confirmed as the first breeding record for Ghana! Rufous-sided Broadbill: A smart male performed admirably as it carried out its acrobatic display flight with amazing mechanical whirring sound. Black Dwarf Hornbill: An amazing 4 different birds seen throughout the tour! Black Bee-Eater: A very understated name for such a fantastically stunning bird! This Yellow-headed Picathartes taken by tour participant Peter Alfrey was one of five birds seen and voted bird of the trip!! WISE BIRDING HOLIDAYS LTD – GHANA: Picathartes to Plover Tour, November 2013 Monday 18th November: Arrive Accra The whole group arrived on time into Accra’s Kotoka Airport. Here, we boarded our spacious air-conditioned coach which was to be our transport for the next 15 days. After about 30 minutes we arrived at our beach hotel and were all soon checked in to our rooms. Everyone was tired, but full of anticipation for our first full days’ birding in Ghana. It was straight to bed for some whereas others were in need of a cool beer before sleep! Tuesday 19th November: Sakumono Lagoon / Winneba Plains It was a relaxed start today with a brief look at the sea before breakfast where the highlights were a few Royal Terns and a Caspian Tern along with our first Copper Sunbird and Laughing Dove in the hotel gardens. -

A Molecular Phylogeny of the Peacock-Pheasants (Galliformes: Polyplectron Spp.) Indicates Loss and Reduction of Ornamental Traits and Display Behaviours
Biological Journal of the Linnean Society (2001), 73: 187–198. With 3 figures doi:10.1006/bijl.2001.0536, available online at http://www.idealibrary.com on A molecular phylogeny of the peacock-pheasants (Galliformes: Polyplectron spp.) indicates loss and reduction of ornamental traits and display behaviours REBECCA T. KIMBALL1,2∗, EDWARD L. BRAUN1,3, J. DAVID LIGON1, VITTORIO LUCCHINI4 and ETTORE RANDI4 1Department of Biology, University of New Mexico, Albuquerque, NM 87131, USA 2Department of Evolution, Ecology and Organismal Biology, Ohio State University, Columbus, OH 43210, USA 3Department of Plant Biology, Ohio State University, Columbus, OH 43210, USA 4Istituto Nazionale per la Fauna Selvatica, via Ca` Fornacetta 9, 40064 Ozzano dell’Emilia (BO), Italy Received 4 September 2000; accepted for publication 3 March 2001 The South-east Asian pheasant genus Polyplectron is comprised of six or seven species which are characterized by ocelli (ornamental eye-spots) in all but one species, though the sizes and distribution of ocelli vary among species. All Polyplectron species have lateral displays, but species with ocelli also display frontally to females, with feathers held erect and spread to clearly display the ocelli. The two least ornamented Polyplectron species, one of which completely lacks ocelli, have been considered the primitive members of the genus, implying that ocelli are derived. We examined this hypothesis phylogenetically using complete mitochondrial cytochrome b and control region sequences, as well as sequences from intron G in the nuclear ovomucoid gene, and found that the two least ornamented species are in fact the most recently evolved. Thus, the absence and reduction of ocelli and other ornamental traits in Polyplectronare recent losses. -

Defining a Monophyletic Cardinalini
Available online at www.sciencedirect.com Molecular Phylogenetics and Evolution 45 (2007) 1014–1032 www.elsevier.com/locate/ympev Defining a monophyletic Cardinalini: A molecular perspective John Klicka a,*, Kevin Burns b, Garth M. Spellman a,1 a Barrick Museum of Natural History, Box 454012, University of Nevada Las Vegas, 4505 Maryland, Parkway, Las Vegas, NV 89154-4012, USA b Department of Biology, San Diego State University, San Diego, CA 92182-4614, USA Received 28 March 2007; revised 29 June 2007; accepted 10 July 2007 Available online 19 July 2007 Abstract Within the New World nine-primaried oscine assemblage, feeding morphology and behavior have long been used as a guideline for assigning membership to subgroups. For example, birds with stout, conical bills capable of crushing heavy seeds have generally been placed within the tribe Cardinalini (cardinal-grosbeaks). Many workers have tried to characterize this group more definitively, using a variety of morphological characters; however, the characters used often conflicted with one another. Previous molecular studies addressing the monophyly of Cardinalini have had only limited sampling within the group. In this study, we analyze mtDNA sequence data from all genera and 34 of the 42 Cardinalini species (sensu [Sibley, C.G., Monroe, B.L., 1990. Distribution and Taxonomy of the Birds of the World, Yale University Press, New Haven, CT]) to address the monophyly of the group and to reconstruct the most com- plete phylogeny of this tribe published to date. We found strong support for a redefined Cardinalini that now includes some members previously placed within Thraupini (tanagers; the genera Piranga, Habia, Chlorothraupis, and Amaurospiza) and some members previ- ously placed within the Parulini (wood-warblers; the genus Granatellus).