Habitat Selection of "Mad Cocks" of the Western Capercaillies Tetrao Urogallus (Galliformes: Phasianidae) from the Fringe of the Range: a Case Study from Rila Mts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nogth AMERICAN BIRDS
CHECK-LIST OF NOgTH AMERICAN BIRDS The Speciesof Birds of North America from the Arctic through Panama, Including the West Indies and Hawaiian Islands PREPARED BY THE COMMITTEE ON CLASSIFICATION AND NOMENCLATURE OF THE AMERICAN ORNITHOLOGISTS' UNION SEVENTH EDITION 1998 Zo61ogical nomenclature is a means, not an end, to Zo61ogical Science PUBLISHED BY THE AMERICAN ORNITHOLOGISTS' UNION 1998 Copyright 1998 by The American Ornithologists' Union All rights reserved, except that pages or sections may be quoted for research purposes. ISBN Number: 1-891276-00-X Preferred citation: American Ornithologists' Union. 1983. Check-list of North American Birds. 7th edition. American Ornithologists' Union, Washington, D.C. Printed by Allen Press, Inc. Lawrence, Kansas, U.S.A. CONTENTS DEDICATION ...................................................... viii PREFACE ......................................................... ix LIST OF SPECIES ................................................... xvii THE CHECK-LIST ................................................... 1 I. Tinamiformes ............................................. 1 1. Tinamidae: Tinamous .................................. 1 II. Gaviiformes .............................................. 3 1. Gaviidae: Loons ....................................... 3 III. Podicipediformes.......................................... 5 1. Podicipedidae:Grebes .................................. 5 IV. Procellariiformes .......................................... 9 1. Diomedeidae: Albatrosses ............................. -

Birds of Bharatpur – Check List
BIRDS OF BHARATPUR – CHECK LIST Family PHASIANIDAE: Pheasants, Partridges, Quail Check List BLACK FRANCOLIN GREY FRANCOLIN COMMON QUAIL RAIN QUAIL JUNGLE BUSH QUAIL YELLOW-LEGGED BUTTON QUAIL BARRED BUTTON QUAIL PAINTED SPURFOWL INDIAN PEAFOWL Family ANATIDAE: Ducks, Geese, Swans GREATER WHITE-FRONTED GOOSE GREYLAG GOOSE BAR-HEADED GOOSE LWSSER WHISTLING-DUCK RUDDY SHELDUCK COMMON SHELDUCK COMB DUCK COTTON PYGMY GOOSE MARBLED DUCK GADWALL FALCATED DUCK EURASIAN WIGEON MALLARD SPOT-BILLED DUCK COMMON TEAL GARGANEY NORTHERN PINTAIL NORTHERN SHOVELER RED-CRESTED POCHARD COMMON POCHARD FERRUGINOUS POCHARD TUFTED DUCK BAIKAL TEAL GREATER SCAUP BAER’S POCHARD Family PICIDAE: Woodpeckers EURASIAN WRYNECK BROWN-CAPPED PYGMY WOODPECKER YELLOW-CROWNED WOODPECKER BLACK-RUMPED FLAMBACK Family CAPITONIDAE: Barbets BROWN-HEADED BARBET COPPERSMITH BARBET Family UPUPIDAE: Hoopoes COMMON HOOPOE Family BUCEROTIDAE: Hornbills INDAIN GREY HORNBILL Family CORACIIDAE: Rollers or Blue Jays EUROPEAN ROLLER INDIAN ROLLER Family ALCEDINIDAE: Kingfisher COMMON KINGFISHER STORK-BILLED KINGFISHER WHITE-THROATED KINGFISHER BLACK-CAPPED KINGFISHER PIED KINGFISHER Family MEROPIDAE: Bee-eaters GREEN BEE-EATER BLUE-CHEEKED BEE-EATER BLUE-TAILED BEE-EATER Family CUCULIDAE: Cuckoos, Crow-pheasants PIED CUCKOO CHESTNUT-WINGED CUCKOO COMMON HAWK CUCKOO INDIAN CUCKOO EURASIAN CUCKOO GREY-BELLIED CUCKOO PLAINTIVE CUCKOO DRONGO CUCKOO ASIAN KOEL SIRKEER MALKOHA GREATER COUCAL LESSER COUCAL Family PSITTACIDAS: Parrots ROSE-RINGED PARAKEET PLUM-HEADED PARKEET Family APODIDAE: -

The Impact of Wind Energy Facilities on Grouse: a Systematic Review
Journal of Ornithology (2020) 161:1–15 https://doi.org/10.1007/s10336-019-01696-1 REVIEW The impact of wind energy facilities on grouse: a systematic review Joy Coppes1 · Veronika Braunisch1,2 · Kurt Bollmann3 · Ilse Storch4 · Pierre Mollet5 · Veronika Grünschachner‑Berger6,7 · Julia Taubmann1,4 · Rudi Suchant1 · Ursula Nopp‑Mayr8 Received: 17 January 2019 / Revised: 1 July 2019 / Accepted: 18 July 2019 / Published online: 1 August 2019 © Deutsche Ornithologen-Gesellschaft e.V. 2019 Abstract There is increasing concern about the impact of the current boom in wind energy facilities (WEF) and associated infra- structure on wildlife. However, the direct and indirect efects of these facilities on the mortality, occurrence and behaviour of rare and threatened species are poorly understood. We conducted a literature review to examine the potential impacts of WEF on grouse species. We studied whether grouse (1) collide with wind turbines, (2) show behavioural responses in relation to wind turbine developments, and (3) if there are documented efects of WEF on their population sizes or dynam- ics. Our review is based on 35 sources, including peer-reviewed articles as well as grey literature. Efects of wind turbine facilities on grouse have been studied for eight species. Five grouse species have been found to collide with wind turbines, in particular with the towers. Fifteen studies reported behavioural responses in relation to wind turbine facilities in grouse (seven species), including spatial avoidance, displacement of lekking or nesting sites, or the time invested in breeding vs. non-breeding behaviour. Grouse were afected at up to distances of 500 m by WEF infrastructure, with indications of efects also at bigger distances. -

Wild Turkey Education Guide
Table of Contents Section 1: Eastern Wild Turkey Ecology 1. Eastern Wild Turkey Quick Facts………………………………………………...pg 2 2. Eastern Wild Turkey Fact Sheet………………………………………………….pg 4 3. Wild Turkey Lifecycle……………………………………………………………..pg 8 4. Eastern Wild Turkey Adaptations ………………………………………………pg 9 Section 2: Eastern Wild Turkey Management 1. Wild Turkey Management Timeline…………………….……………………….pg 18 2. History of Wild Turkey Management …………………...…..…………………..pg 19 3. Modern Wild Turkey Management in Maryland………...……………………..pg 22 4. Managing Wild Turkeys Today ……………………………………………….....pg 25 Section 3: Activity Lesson Plans 1. Activity: Growing Up WILD: Tasty Turkeys (Grades K-2)……………..….…..pg 33 2. Activity: Calling All Turkeys (Grades K-5)………………………………..…….pg 37 3. Activity: Fit for a Turkey (Grades 3-5)…………………………………………...pg 40 4. Activity: Project WILD adaptation: Too Many Turkeys (Grades K-5)…..…….pg 43 5. Activity: Project WILD: Quick, Frozen Critters (Grades 5-8).……………….…pg 47 6. Activity: Project WILD: Turkey Trouble (Grades 9-12………………….……....pg 51 7. Activity: Project WILD: Let’s Talk Turkey (Grades 9-12)..……………..………pg 58 Section 4: Additional Activities: 1. Wild Turkey Ecology Word Find………………………………………….…….pg 66 2. Wild Turkey Management Word Find………………………………………….pg 68 3. Turkey Coloring Sheet ..………………………………………………………….pg 70 4. Turkey Coloring Sheet ..………………………………………………………….pg 71 5. Turkey Color-by-Letter……………………………………..…………………….pg 72 6. Five Little Turkeys Song Sheet……. ………………………………………….…pg 73 7. Thankful Turkey…………………..…………………………………………….....pg 74 8. Graph-a-Turkey………………………………….…………………………….…..pg 75 9. Turkey Trouble Maze…………………………………………………………..….pg 76 10. What Animals Made These Tracks………………………………………….……pg 78 11. Drinking Straw Turkey Call Craft……………………………………….….……pg 80 Section 5: Wild Turkey PowerPoint Slide Notes The facilities and services of the Maryland Department of Natural Resources are available to all without regard to race, color, religion, sex, sexual orientation, age, national origin or physical or mental disability. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

Partridges, Quails, Pheasants and Turkeys Phasianidae Vigors, 1825: Zoological Journal 2: 402 – Type Genus Phasianus Linnaeus, 1758
D .W . .5 / DY a 5D t w[ { wt Ç"" " !W5 í ÇI &'(' / b ù b a L w 5 ! ) " í "* " Ç t+ t " h " * { b ù" t* &)/&0 Order GALLIFORMES: Game Birds and Allies The order of galliform taxa in Checklist Committee (1990) appears to have been based on Peters (1934). Johnsgard (1986) synthesised available data, came up with similar groupings of taxa, and produced a dendrogram indicating that turkeys (Meleagridinae) were the most primitive (outside Cracidae and Megapodiidae), with grouse (Tetraoninae), guineafowl (Numidinae), New World quails (Odontophorinae) and pheasants and kin (Phasianinae) successively more derived. Genetic evidence (DNA-hybridisation data) provided by Sibley & Ahlquist (1990) suggested Odontophorinae were the most basal phasianoids and guineafowl the next most basal group. A basal position of the New World quails among phasianoids has been supported by other genetic data (Kimball et al. 1999, Armstrong et al. 2001). A recent analysis based on morphological characters (Dyke et al. 2003) found support for megapodes as the most basal group in the order, then Cracidae, then Phasianidoidea, and within the latter, Numididae the most basal group. In contrast to the above genetic-based analyses, Dyke et al. (2003) found the Odontophorinae to be the most derived group within the order. A recent analysis using both mitochondrial ND2 and cytochrome-b DNA sequences, however, reinforces the basal position of the Odontophorinae (Pereira & Baker 2006). Here we follow a consensus of the above works and place Odontophorinae basal in the phasianids. Worthy & Holdaway (2002) considered that Cheeseman’s (1891) second-hand record of megapodes from Raoul Island, Kermadec Group, before the 1870 volcanic eruption has veracity. -

Aberrant Plumages in Grebes Podicipedidae
André Konter Aberrant plumages in grebes Podicipedidae An analysis of albinism, leucism, brown and other aberrations in all grebe species worldwide Aberrant plumages in grebes Podicipedidae in grebes plumages Aberrant Ferrantia André Konter Travaux scientifiques du Musée national d'histoire naturelle Luxembourg www.mnhn.lu 72 2015 Ferrantia 72 2015 2015 72 Ferrantia est une revue publiée à intervalles non réguliers par le Musée national d’histoire naturelle à Luxembourg. Elle fait suite, avec la même tomaison, aux TRAVAUX SCIENTIFIQUES DU MUSÉE NATIONAL D’HISTOIRE NATURELLE DE LUXEMBOURG parus entre 1981 et 1999. Comité de rédaction: Eric Buttini Guy Colling Edmée Engel Thierry Helminger Mise en page: Romain Bei Design: Thierry Helminger Prix du volume: 15 € Rédaction: Échange: Musée national d’histoire naturelle Exchange MNHN Rédaction Ferrantia c/o Musée national d’histoire naturelle 25, rue Münster 25, rue Münster L-2160 Luxembourg L-2160 Luxembourg Tél +352 46 22 33 - 1 Tél +352 46 22 33 - 1 Fax +352 46 38 48 Fax +352 46 38 48 Internet: http://www.mnhn.lu/ferrantia/ Internet: http://www.mnhn.lu/ferrantia/exchange email: [email protected] email: [email protected] Page de couverture: 1. Great Crested Grebe, Lake IJssel, Netherlands, April 2002 (PCRcr200303303), photo A. Konter. 2. Red-necked Grebe, Tunkwa Lake, British Columbia, Canada, 2006 (PGRho200501022), photo K. T. Karlson. 3. Great Crested Grebe, Rotterdam-IJsselmonde, Netherlands, August 2006 (PCRcr200602012), photo C. van Rijswik. Citation: André Konter 2015. - Aberrant plumages in grebes Podicipedidae - An analysis of albinism, leucism, brown and other aberrations in all grebe species worldwide. Ferrantia 72, Musée national d’histoire naturelle, Luxembourg, 206 p. -

An Assessment of the Risk Associated with the Movement Turkeys To
United States Department of Agriculture Animal and Plant Health Inspection Service Veterinary Services Science, Technology, An Assessment of the Risk Associated with the and Analysis Services Movement Turkeys to Market Into, Within, and Center for Epidemiology and Animal Health Out of a Control Area During a Highly 2150 Centre Avenue Pathogenic Avian Influenza Outbreak in the Building B Fort Collins, CO 80526 United States March 2017 FIRST DRAFT September 2017 SECOND REVIEW January 2018 THIRD REVIEW October 2018 FINAL REVIEW AND CLEARANCE A Collaboration between the Turkey Sector Working Group, the University of Minnesota’s Secure Food Systems Team, and USDA:APHIS:VS:CEAH UNITED STATES DEPARTMENT OF AGRICULTURE ANIMAL AND PLANT HEALTH INSPECTION SERVICE VETERINARY SERVICES CENTER FOR EPIDEMIOLOGY AND ANIMAL HEALTH Turkeys to Market Risk Assessment Suggested bibliographic citation for this report: Carol Cardona, Carie Alexander, Justin Bergeron, Peter Bonney, Marie Culhane, Timothy Goldsmith, David Halvorson, Eric Linskens, Sasidhar Malladi, Amos Ssematimba, Emily Walz, Todd Weaver, Jamie Umber. An Assessment of the Risk Associated with the Movement of Turkeys to Market Into, Within, and Out of a Control Area during a Highly Pathogenic Avian Influenza Outbreak in the United States. Collaborative agreement between USDA:APHIS:VS and University of Minnesota Center for Secure Food Systems. Fort Collins, CO. October 2018. 217 pgs. This document was developed through the Continuity of Business / Secure Food Supply Plans / Secure Poultry Supply -

Update of the Situation of the Cantabrian Capercaillie Tetrao Urogallus Cantabricus: an Ongoing Decline Maria José Bañuelos & Mario Quevedo
Grouse News 25 Newsletter of Grouse Specialist Group Update of the situation of the Cantabrian capercaillie Tetrao urogallus cantabricus: an ongoing decline Maria José Bañuelos & Mario Quevedo In a previous number of Grouse News (Bañuelos et al. 2004) we reported the drastic decline that Cantabrian capercaillie Tetrao urogallus cantabricus had apparently suffered in two decades, from the early 1980’s to 2000 - 2001. Based on extensive evaluation of lek occupancy, about 50% of the display areas had been deserted, and the number of remaining cocks was roughly estimated at 300. Accordingly, Cantabrian capercaillie was listed as endangered in Spain, and became the only subspecies of capercaillie qualifying as endangered according to IUCN criteria (Storch et al. 2006). This capercaillie population occupies a very southerly range for a tetraonid (Quevedo et al. 2006a), and has recently been identified as an evolutionary significant unit because of its unique ecological and genetic characteristics (Rodríguez- Muñoz et al. 2007). Between 2005 and 2007 a new extensive lek survey was performed in the northern watershed of the range (Asturias), over a territory that comprises more than 50% of the population. Almost all known display areas (N=364) were repeatedly surveyed during the lekking season (April-May). Surveys were performed during the day, looking for signs such as feathers, fresh droppings or footprints, so that results were mainly presence-absence data. Occupancy surveys during the day were mostly chosen over more traditional lek counts at dawn to minimize disturbances, but also because the previous survey (2000/2001) showed that less than 10% of the occupied sites had more than one cock. -
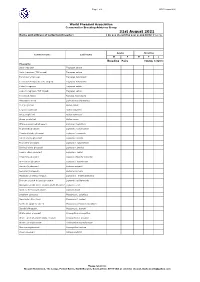
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor -

National Biodiversity Strategy and Action Plan of Georgia
Biodiversity Strategy and Action Plan - Georgia – Tbilisi, 2005 Foreword Georgia signed the Convention on Biological Diversity in 1994, thus accepting responsibility to safeguard the nation’s rich diversity of plant, animal, and microbial life, to begin using biological resources in sustainable way, and to ensure equitable sharing of benefits from biodiversity. Later the country joined other conventions including the Convention on Climate Change, the Ramsar Convention on Wetlands, CITES and the Bonn Convention. As a signatory to these important international environmental treaties, Georgia enters the world scene with the potential for joining the most advanced nations in the field of environmental protection. At the present moment of transition, Georgia has a unique opportunity to use the early experiences of other countries, and avoid irreversible changes in the quality of its environment. The national legislation on environmental protection adopted over the past few years provides an adequate legal basis for this, although further elaboration and reinforcement of the existing legislation is needed. With the Ministry of Environment being currently reorganised and assuming broader responsibilities, Georgia’s institutional arrangements for environmental protection already has the necessary structure for improving the quality of the environment throughout the country. The role of non-governmental groups has been very important in resolving problems related to nature conservation. Georgia has shown an excellent example of co-operation between governmental and non-governmental organizations in the field of environment, and particularly in the field of biodiversity conservation. After signing the Convention on Biological Diversity, the Georgian Government immediately acted to develop a Biodiversity Country Study, in partnership with UNEP, and implemented by NACRES, a local conservation organisation. -

Complete Mitochondrial Genome of the Western Capercaillie Tetrao Urogallus (Phasianidae, Tetraoninae)
Zootaxa 4550 (4): 585–593 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4550.4.9 http://zoobank.org/urn:lsid:zoobank.org:pub:12E18262-0DCA-403A-B047-82CFE5E20373 Complete mitochondrial genome of the Western Capercaillie Tetrao urogallus (Phasianidae, Tetraoninae) GAËL ALEIX-MATA1,5, FRANCISCO J. RUIZ-RUANO2, JESÚS M. PÉREZ1, MATHIEU SARASA3 & ANTONIO SÁNCHEZ4 1Department of Animal and Plant Biology and Ecology, Jaén University, Campus Las Lagunillas, E-23071, Jaén, Spain. E-mail: [email protected] 2Departamento de Genética, Facultad de Ciencias, Universidad de Granada, Avda. Fuentenueva, 18071 Granada, Spain. 3BEOPS, 1 Esplanade Compans Caffarelli, 31000 Toulouse, France 4Department of Experimental Biology, Jaén University, Campus Las Lagunillas, E-23071, Jaén, Spain 5Corresponding author Gaël Aleix-Mata: [email protected] ORCID: 0000-0002-7429-4051 Francisco J. Ruíz-Ruano: [email protected] ORCID: 0000-0002-5391-301X Jesús M. Pérez: [email protected] ORCID: 0000-0001-9159-0365 Mathieu Sarasa: [email protected] ORDCID: 0000-0001-9067-7522 Antonio Sánchez: [email protected] ORCID: 0000-0002-6715-8158 Abstract The Western Capercaillie (Tetrao urogallus) is a galliform bird of boreal climax forests from Scandinavia to eastern Sibe- ria, with a fragmented population in southwestern Europe. We extracted the DNA of T. urogallus aquitanicus and obtained the complete mitochondrial genome (mitogenome) sequence by combining Illumina and Sanger sequencing sequence da- ta. The mitochondrial genome of T. urogallus is 16,683 bp long and is very similar to that of Lyrurus tetrix (16,677 bp).