Steroidal Saponins from the Genus Allium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sustainable Crop Protection
SUSTAINABLE CROP PROTECTION Results from the Pesticide Risk Reduction Program An Integrated Approach to Management of Leek Moth 10 years of collaborative research, development and knowledge transfer Photo credits: A. Brauner Figure 1: Leek Moth (Acrolepiopsis assectella) at key developmental stages Background Establishing Base Knowledge – The leek moth, Acrolepiopsis assectella, (Figure 1) is an invasive Leek moth life history in Canada alien species from Europe that causes damage to onions, leeks As a new pest to Canada, initial work focused on determining and garlic. Larvae cause damage when they penetrate the young the life cycle of the pest under Eastern Ontario conditions. leaves and flowers of the crop in order to feed (Figure 2). This Pheromone traps were used to monitor flight patterns and feeding weakens and withers the plant reducing the value of the numbers of adult leek moth during the growing season, while crop, and in some cases renders it unmarketable. First detected field populations of leek moth larvae and pupae were estimated in Eastern Ontario in 1993 and Quebec in 2001, leek moth has through destructive plant sampling. Assessment of garlic scapes rapidly expanded its range and, as of 2013, was detected as far as and bulbs were used to estimate damage to marketable products. Southwestern Ontario, Prince Edward Island and New York State. It was found that the development of leek moth populations in The Pesticide Risk Reduction Program of Agriculture and Canada requires 441.7 day-degrees from egg to adult. In the Agri-Food Canada’s (AAFC) Pest Management Centre has, over Ottawa area, pheromone trap data indicated that there are three the past ten years, supported several projects towards the flight periods: a spring flight of adults that overwintered, an early development of an Integrated Pest Management (IPM) strategy summer flight of 1st generation adults and a late summer flight of to address this emerging pest issue. -

Karyologická Variabilita Vybraných Taxonů Rodu Allium V Evropě Alena
UNIVERZITA PALACKÉHO V OLOMOUCI Přírodov ědecká fakulta Katedra botaniky Karyologická variabilita vybraných taxon ů rodu Allium v Evrop ě Diplomová práce Alena VÁ ŇOVÁ obor: T ělesná výchova - Biologie Prezen ční studium Vedoucí práce: RNDr. Martin Duchoslav, Ph.D. Olomouc 2011 Prohlašuji, že jsem zadanou diplomovou práci vypracovala samostatn ě s použitím citované literatury a konzultací. V Olomouci dne: 14.1.2011 ................................................. Pod ěkování Ráda bych pod ěkovala všem, co mi v jakémkoli ohledu pomohli. P ředevším svému vedoucímu diplomové práce RNDr. Martinu Duchoslavovi, PhD., a to nejen za cenné rady a pomoc p ři práci, ale p ředevším za velké množství trp ělivosti. Stejn ě tak d ěkuji Mgr. Míše Jandové za veškerý čas, který mi v ěnovala, Tereze P ěnkavové za pomoc ve skleníku a odd ělení fytopatologie za možnost využívat jejich laborato ří. Samoz řejm ě mé díky pat ří i všem blízkým, kte ří m ě po dobu studia podporovali. Bibliografická identifikace Jméno a p říjmení autora : Alena Vá ňová Název práce : Karyologická variabilita vybraných taxon ů rodu Allium v Evrop ě. Typ práce : Diplomová Pracovišt ě: Katedra botaniky, P řírodov ědecká fakulta Univerzity Palackého v Olomouci Vedoucí práce : RNDr. Martin Duchoslav, Ph.D. Rok obhajoby práce : 2011 Abstrakt : Diplomová práce m ěla za cíl postihnout karyologickou variabilitu (chromozomový po čet, ploidní úrove ň a DNA-ploidní úrove ň) a velikost jaderné DNA (2C) vybraných taxon ů rodu Allium pro populace získané z různých částí Evropy. Celkov ě bylo pomocí karyologických metod prov ěř eno 550 jedinc ů u 14 taxon ů rodu Allium : A. albidum, A. -

Evaluation of Larvicidal Activity of the Essential Oil of Allium Macrostemon
Liu et al. Parasites & Vectors 2014, 7:184 http://www.parasitesandvectors.com/content/7/1/184 RESEARCH Open Access Evaluation of larvicidal activity of the essential oil of Allium macrostemon Bunge and its selected major constituent compounds against Aedes albopictus (Diptera: Culicidae) Xin Chao Liu1, Qiyong Liu2, Ligang Zhou3 and Zhi Long Liu1* Abstract Background: During the screening programme for new agrochemicals from Chinese medicinal herbs and local wild plants, the essential oil of dried bulbs of Allium macrostemon Bunge (Liliaceae) was found to possess larvicidal activity against mosquitoes. The aim of this research was to determine the larvicidal activity of the essential oil and its major constituent compounds against the larvae of the Culicidae mosquito, Aedes albopictus. Methods: Essential oil of A. macrostemon was obtained by hydrodistillation and analyzed by gas chromatography (GC) and gas chromaotography-mass spectrometry (GC-MS). The activity of the essential oil and its two major constituents were evaluated, using World Health Organization (WHO) procedures, against the fourth instar larvae of Ae. albopictus for 24 h and larval mortalities were recorded at various essential oil/compound concentrations ranging from 9.0 - 150 μg/ml. Results: The essential oil of A. macrostemon exhibited larvicidal activity against the early fourth instar larvae of Ae. albopictus with an LC50 value of 72.86 μg/ml. The two constituent compounds, dimethyl trisulfide and methyl propyl disulfide possessed strong larvicidal activity against the early fourth instar larvae of Ae. albopictus with LC50 values of 36.36 μg/ml and 86.16 μg/ml, respectively. Conclusion: The results indicated that the essential oil of A. -

Allium Leaf Miner Is Spreading Around the UK at a Steady Rate with Clusters of Infestation Around the Midlands and London
Allium leaf miner is spreading around the UK at a steady rate with clusters of infestation around the Midlands and London. The pest is most likely to cause problems in leeks where it can wipe out an entire crop September to December is the time when most damage is caused in leek crops. It will be necessary to cover leeks from late August to late December to protect them if you are growing in a problem area People who currently took no action against this pest will cover their leeks in the future, now they are aware of what it is More needs to be done to raise awareness so that people are able to identify and prevent this pest Allium leaf miner is a serious pest that affects all allium crops. It first arrived in the Midlands in 2002, and since then, has rapidly spread around the country. Garden Organic last did a survey of allium leaf miner with the Organic Growers’ Alliance in 2011. The survey showed that there were many siting’s around the Midlands but it had spread to other areas of the UK as well. We suspected that it has spread much further now, so have repeated the survey to find out which areas it is now affecting. Appearance You are most likely to notice the larvae of the allium leaf miner as a small creamy maggot burrowing into the plants. You may also notice the pupae which are shiny brown and about the size of a grain of rice. Be careful not to confuse with the leek moth caterpillar which has a brown head and distinct legs. -

Acrolepiopsis Assectella
Acrolepiopsis assectella Scientific Name Acrolepiopsis assectella (Zeller, 1893) Synonym: Lita vigeliella Duponchel, 1842 Common Name Leek moth, onion leafminer Type of Pest Moth Taxonomic Position Class: Insecta, Order: Lepidoptera, Family: Acrolepiidae Figures 1 & 2. Adult male (top) and female (bottom) Reason for Inclusion of A. assectella. Scale bar is 1 mm (© Jean-François CAPS Community Suggestion Landry, Agriculture & Agri-Food Canada, 2007). Pest Description Eggs: “Roughly oval in shape with raised reticulated sculpturing; iridescent white” (Carter, 1984). Eggs are 0.5 by 1 0.2 mm (< /16 in) (USDA, 1960). Larvae: “Head yellowish brown, sometimes with reddish brown maculation; body yellowish green; spiracles surrounded by sclerotised rings, on abdominal segments coalescent with SD pinacula, these grayish brown; prothoracic and anal plates yellow with brown maculation; thoracic legs yellowish brown’ crochets of abdominal prologs arranged in uniserial circles, each enclosing a short, longitudinal row of 3–5 crochets” 1 (Carter, 1984). Larvae are about 13 to 14 mm (approx. /2 in) long (McKinlay, 1992). Pupae: “Reddish brown; abdominal spiracles on raised tubercles; cremaster abruptly terminated, dorsal lobe with a Figure 3. A. assectella larvae rugose plate bearing eight hooked setae, two rounded ventral on stem of elephant garlic lobes each bearing four hooked setae” (Carter, 1984). The (eastern Ontario, June 2000) (© 1 cocoon is 7 mm (approx. /4 in) long (USDA, 1960). “The Jean-François Landry, cocoon is white in colour and is composed of a loose net-like Agriculture & Agri-Food Canada, 2007). structure” (CFIA, 2012). Last updated: August 23, 2016 9 Adults: “15 mm [approx. /16 in wingspan]. Forewing pale brown, variably suffused with blackish brown; terminal quarter sprinkled with white scales; a distinct triangular white spot on the dorsum near the middle. -

ISTA List of Stabilized Plant Names 7Th Edition
ISTA List of Stabilized Plant Names th 7 Edition ISTA Nomenclature Committee Chair: Dr. M. Schori Published by All rights reserved. No part of this publication may be The Internation Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted Zürichstr. 50, CH-8303 Bassersdorf, Switzerland in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2020 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 ISTA List of Stabilized Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 2 7th Edition ISTA List of Stabilized Plant Names Content Preface .......................................................................................................................................................... 4 Acknowledgements ....................................................................................................................................... 6 Symbols and Abbreviations .......................................................................................................................... -

INDEX for 2011 HERBALPEDIA Abelmoschus Moschatus—Ambrette Seed Abies Alba—Fir, Silver Abies Balsamea—Fir, Balsam Abies
INDEX FOR 2011 HERBALPEDIA Acer palmatum—Maple, Japanese Acer pensylvanicum- Moosewood Acer rubrum—Maple, Red Abelmoschus moschatus—Ambrette seed Acer saccharinum—Maple, Silver Abies alba—Fir, Silver Acer spicatum—Maple, Mountain Abies balsamea—Fir, Balsam Acer tataricum—Maple, Tatarian Abies cephalonica—Fir, Greek Achillea ageratum—Yarrow, Sweet Abies fraseri—Fir, Fraser Achillea coarctata—Yarrow, Yellow Abies magnifica—Fir, California Red Achillea millefolium--Yarrow Abies mariana – Spruce, Black Achillea erba-rotta moschata—Yarrow, Musk Abies religiosa—Fir, Sacred Achillea moschata—Yarrow, Musk Abies sachalinensis—Fir, Japanese Achillea ptarmica - Sneezewort Abies spectabilis—Fir, Himalayan Achyranthes aspera—Devil’s Horsewhip Abronia fragrans – Sand Verbena Achyranthes bidentata-- Huai Niu Xi Abronia latifolia –Sand Verbena, Yellow Achyrocline satureoides--Macela Abrus precatorius--Jequirity Acinos alpinus – Calamint, Mountain Abutilon indicum----Mallow, Indian Acinos arvensis – Basil Thyme Abutilon trisulcatum- Mallow, Anglestem Aconitum carmichaeli—Monkshood, Azure Indian Aconitum delphinifolium—Monkshood, Acacia aneura--Mulga Larkspur Leaf Acacia arabica—Acacia Bark Aconitum falconeri—Aconite, Indian Acacia armata –Kangaroo Thorn Aconitum heterophyllum—Indian Atees Acacia catechu—Black Catechu Aconitum napellus—Aconite Acacia caven –Roman Cassie Aconitum uncinatum - Monkshood Acacia cornigera--Cockspur Aconitum vulparia - Wolfsbane Acacia dealbata--Mimosa Acorus americanus--Calamus Acacia decurrens—Acacia Bark Acorus calamus--Calamus -

Evaluation of Insecticide Chemistries Against the Leek Moth (Lepidoptera: Acrolepiidae), a New Pest in North America Author(S): Daniel L
Evaluation of Insecticide Chemistries Against the Leek Moth (Lepidoptera: Acrolepiidae), a New Pest in North America Author(s): Daniel L. Olmstead and Anthony M. Shelton Source: Florida Entomologist, 95(4):1127-1131. 2012. Published By: Florida Entomological Society URL: http://www.bioone.org/doi/full/10.1653/024.095.0443 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Olmstead & Shelton: Chemical Control of the Leek Moth 1127 EVALUATION OF INSECTICIDE CHEMISTRIES AGAINST THE LEEK MOTH (LEPIDOPTERA: ACROLEPIIDAE), A NEW PEST IN NORTH AMERICA DANIEL L. OLMSTEAD* AND ANTHONY M. SHELTON New York State Agricultural Experiment Station, Department of Entomology, Cornell University, 630 West North Street, Geneva, NY 14456, USA *Corresponding author; E-mail: [email protected] Abstract The leek moth, Acrolepiopsis assectella (Zeller), is a newly introduced micro-lepidopteran pest in North America that attacks Allium crops, including onion, leek, and garlic. -
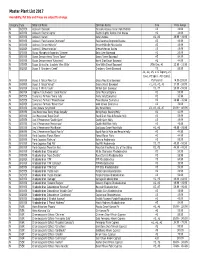
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Survey of Wild Food Plants for Human Consumption in Geçitli (Hakkari, Turkey)
Indian Journal of Traditional Knowledge Vol. 14(2), April 2015, pp. 183-190 Survey of wild food plants for human consumption in Geçitli (Hakkari, Turkey) İdris Kaval1, Lütfi Behçet2 & Uğur Çakilcioğlu3* 1Yuzuncu Yıl University, Department of Biology, Van 65000, Turkey; 2Bingöl University, Department of Biology, Bingöl 12000, Turkey; 3Tunceli University, Pertek Sakine Genç Vocational School, Pertek, Tunceli 62500, Turkey E-mails: [email protected]; [email protected]; [email protected] Received 15 July 2014, revised 22 January 2015 This study aims to record accumulation of knowledge on plants which are used as food by native people of Geçitli (Hakkari, Turkey) that has a rich culture and a very natural environment. In addition, the medical uses of these plants were compiled from the literature. Study area was located on the East of Anatolian diagonal, in the Eastern Anatolia region. Field study was carried out over a period of approximately two years (2008-2010). During this period, 84 vascular plant taxa were collected. The plants were pressed in the field and prepared for identification. A total of 84 food plants belonging to 30 families were identified in the region. In the study being conducted, use of wild plants as food points out interest of people in Geçitli in wild plants. The fact that a large proportion of edible plants are also being used for medicinal purposes indicates that the use of wild plants has a high potential in the region. The present study shows that further ethnobotanical investigations are worthy to be carried out in Turkey, where most of knowledge on popular food plants are still to discover. -

Biological-Control-Programmes-In
Biological Control Programmes in Canada 2001–2012 This page intentionally left blank Biological Control Programmes in Canada 2001–2012 Edited by P.G. Mason1 and D.R. Gillespie2 1Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada; 2Agriculture and Agri-Food Canada, Agassiz, British Columbia, Canada iii CABI is a trading name of CAB International CABI Head Offi ce CABI Nosworthy Way 38 Chauncey Street Wallingford Suite 1002 Oxfordshire OX10 8DE Boston, MA 02111 UK USA Tel: +44 (0)1491 832111 T: +1 800 552 3083 (toll free) Fax: +44 (0)1491 833508 T: +1 (0)617 395 4051 E-mail: [email protected] E-mail: [email protected] Website: www.cabi.org Chapters 1–4, 6–11, 15–17, 19, 21, 23, 25–28, 30–32, 34–36, 39–42, 44, 46–48, 52–56, 60–61, 64–71 © Crown Copyright 2013. Reproduced with the permission of the Controller of Her Majesty’s Stationery. Remaining chapters © CAB International 2013. All rights reserved. No part of this publication may be reproduced in any form or by any means, electroni- cally, mechanically, by photocopying, recording or otherwise, without the prior permission of the copyright owners. A catalogue record for this book is available from the British Library, London, UK. Library of Congress Cataloging-in-Publication Data Biological control programmes in Canada, 2001-2012 / [edited by] P.G. Mason and D.R. Gillespie. p. cm. Includes bibliographical references and index. ISBN 978-1-78064-257-4 (alk. paper) 1. Insect pests--Biological control--Canada. 2. Weeds--Biological con- trol--Canada. 3. Phytopathogenic microorganisms--Biological control- -Canada. -

Alliums of All Shapes & Sizes’
Tips From Trecanna Trecanna Nursery is a family-run plant nursery owned by Mark & Karen Wash and set on the Cornish slopes of the Tamar Valley, specialising in unusual bulbs & perennials, Crocosmias and other South African plants. Each month Mark will write a feature on some of his very favourite plants. Trecanna Nursery is now open from Wednesday to Saturday throughout the year, from 10am to 5pm, (or phone to arrange a visit at other times). There is a wide range of unusual bulbs, herbaceous plants and hardy South African plants including the largest selection of Crocosmia in the South. We are located approx. 2 miles north of Gunnislake. Follow the brown tourist signs from the A390, Callington to Gunnislake road. Tel: 01822 834680. Email: [email protected] Talks to garden clubs and societies. ‘Alliums Of All Shapes & Sizes’ Last year I covered a number of fabulous Alliums that you plant and enjoy in your garden, however as there are so many excellent varieties to choose from, I have decided to look at some more - particularly as May & June is when the vast majority of them burst into flower. The main displays of spring flowering bulbs, including narcissi and most tulips, are just coming to an end in May. The Alliums fulfil a valuable task, bridging the gap between Spring & Summer before many of our herbaceous plants come into their prime. There are a vast array of wild Alliums in existence coming from areas such as Asia, North America and Europe – in fact the wild species number over 700 and with all the hybrids that have been bred over the years the choice is now literally thousands.