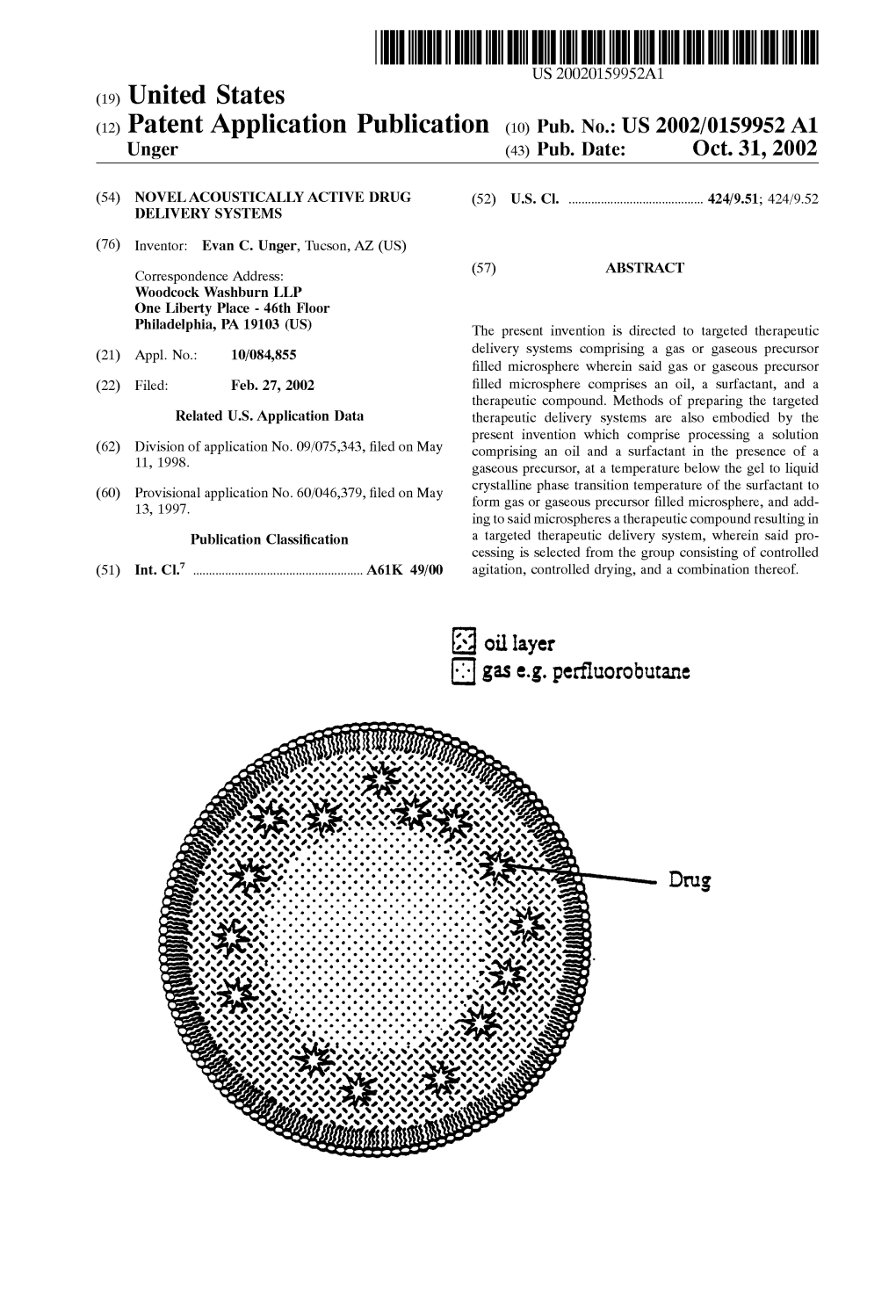
Gas E.G. Perfluorobutane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prepared by 3M
DRAFT ECOTOXICOLOGY AND ENVIRONMENTAL FATE TESTING OF SHORT CHAIN PERFLUOROALKYL COMPOUNDS RELATED TO 3M CHEMISTRIES XXX XX, 2008 Prepared by 3M Page 1 CONFIDENTIAL - SUBJECT TO A PROTECTIVE ORDER ENTERED IN 3M MN01537089 HENNEPIN COUNTY DISTRICT COURT, NO. 27-CV-10-28862 2231.0001 DRAFT TABLE OF CONTENTS Page Introduction (describes propose, goals, document organization .... ) 3 1) Degradation modes 4-27 Basic degradation pathways, Conversion routes from polymer to monomer & degradants or residual intermediates to degradants) 2) Degradants & Intermediates - Phys. Chem properties and test data by section & chapter i) Fluorinated Sulfonic acids and derivatives a) PFBS (PBSK) 28-42 b) PFBSI 43-44 ii) Fluorinated Carboxylates a) TFA 46-55 b) PFPA 56-59 c) PFBA (APFB) 60-65 d) MeFBSAA (MeFBSE Acid, M370) 66-68 iii) Fluorinated Non-ionics a) MeFBSE 70-74 b) MeFBSA 75-80 c) FBSE 81-84 d) FBSA 85-88 e) HxFBSA 89-91 iv) Fluorinated Inerts & volatiles a) PBSF 94-97 b) NFB, C4 hydride, CF3CF2CF2CF2-H 98-99 3) Assessments X Glossary 100-102 References/Endnotes 105- Page 2 CONFIDENTIAL - SUBJECT TO A PROTECTIVE ORDER ENTERED IN 3M MN01537090 HENNEPIN COUNTY DISTRICT COURT, NO. 27-CV-10-28862 2231.0002 DRAFT Introduction to Environmental White Papers on Perfluoroalkyl acids related to 3M chemistries Perfluorochemicals have been commonplace in chemical industry over 50 years but until recently there has been little information on environmental fate and effects avialble in open literature. The following chapters summarize the findings of"list specific C4 intermediates PFBS, PFBSI, PFBA, PFPA, MeFBSAA, TFA, MeFBSE, FBSA, HxFBSA, FBSE, PBSF, NFB " As background, 3M announced on May 16, 2000 the voluntary manufacturing phase out of perfluorooctanyl chemicals which included perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), and PFOS-related chemistries. -

Environmental Management of Firefighting Foam Policy Explanatory Notes Revision 2
Environmental Management of Firefighting Foam Policy Explanatory Notes Revision 2 Prepared by: Incident Response Unit, Department of Environment and Heritage Protection © State of Queensland, 2014-16. The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 3.0 Australia (CC BY) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. For more information on this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Disclaimer This document has been prepared with all due diligence and care, based on the best available information at the time of publication. The department holds no responsibility for any errors or omissions within this document. Any decisions made by other parties based on this document are solely the responsibility of those parties. Information contained in this document is from a number of sources and, as such, does not necessarily represent government or departmental policy. Revision 2.2 – July 2016 www.EHP.qld.gov.au ABN 46 640 294 485 Firefighting Foam Management Policy – Explanatory Notes (Revision 2) Contents 1 Introduction ................................................................................................................................................1 1.1 Background -
C1-C4 Halogenated HC Info 16FEB2018.Xlsx
C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) Feb 28, 2018 Administrative Council Mtg - Printed 2/16/20184:11 PM Reported Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR, mfr literature) TURA TSCA CDR Chemical CAS/Chemica chemical name chemical fire 2015 Chemical Name Pesti- blowing feedstock / supressant / propell- Inventory? TRI? List l Number (synonyms) formula Solvent refriger-ant etchant Other Tier II cide agent intermed-iate flame ant status* retardant insulation for refrigerators, freezers, commercial refrigeration equipment, refrigerated containers and LNG ships; spray foam insulation; insulated metal panels; slabstock and molded flexible foam; refrigerant for chillers; and solvents for metal cleaning and electronics, and circuit flush. https://www.honeywell-blowingagents.com/?document=solstice-lba-technical- HFO-1233zd (E) brochure&download=1 Hydro-fluoro-olefin C H ClF YYY yhttps://www.honeywell-refrigerants.com/americas/product/solstice-zd/ (HFO) 3 2 3 CDR 2016 Honeywell: 100% as propellants and blowing agents for plastics product mfg; R-1233zd CDR Honeywell Solstice® ZD refrigerant, Solstice® 1233zdE, Solstice Blowing Agent; Arkema (current Forane® 1233zd blowing agent for spray PU foam, appliance insulation, etc. and solvent (may Honeywell not be available in US) product) C1-C4 Cat 102687-65-0 1-Chloro-3,3,3-trifluoropropene Y 106‐95‐6 Allyl bromide 2-Propenyl bromide C3H5Br Y y Manufacture of synthetic perfumes, other allyl compounds; Insecticidal fumigant; 1-Propene, 3-bromo- Chemical intermediate in organic synthesis, for resins (copolymer with sulfur dioxide) and fragrances; As a fumigant (if quite volatile) or as a contact poison; C1-C4 Cat Used in the manufacture of plastics and dyestuff. -

Comparative Study of New Environmentally Safe Refrigerant Fluids
Comparative Study of New Environmentally Safe Refrigerant Fluids A thesis submitted to the University of Khartoum in Partial fulfillment of the requirement for the degree of M.Sc in Chemical Engineering By Momin Elhadi Abdalla Mohammed B.Sc. (Honors in Chemical Engineering) (2006) University of Khartoum Supervisor Dr. Ali Abdelrahman Rabah Faculty of Engineering, Department of Chemical Engineering August / 2009 ﺳﻮرة اﻟﺒﻘﺮة Dedication I’m honored to dedicate this thesis to: My Dear Parents My Friends & Humanity I Acknowledgements I would like to express my gratitude to Dr. Ali Abdelrahman Rabah, for his invaluable guidance, experience, motivation and support. His advice helped me think rationally about complex problems and solve them in a correct and yet simple manner. My thanks also go to all the staff at the University Of Khartoum Department Of Chemical Engineering for their logistic supports during my study and research as a graduate student and a teaching assistant staff. Finally, I sincerely thank all my friends who have provided me with so much help to make this work possible. II TABLE OF CONTENTS Dedication ..........................................................................................................................I Acknowledgement ......................................................................................................................... II Abstract ………............................................................................................................ VI Abstract (Arabic) ........................................................................................................................ -

Hydrogen Fluoride) Production from Halon 1301 and Alternative Gaseous Fire Suppressants a Review
Acid gas (hydrogen fluoride) production from Halon 1301 and alternative gaseous fire suppressants A Review John A. Hiltz DRDC – Atlantic Research Centre Defence Research and Development Canada Scientific Report DRDC-RDDC-2015-R036 March 2015 IMPORTANT INFORMATIVE STATEMENTS This work was carried out as part of the Naval Platforms Project – 01 ea. © Her Majesty the Queen in Right of Canada, as represented by the Minister of National Defence, 2015 © Sa Majesté la Reine (en droit du Canada), telle que représentée par le ministre de la Défense nationale, 2015 Abstract …….. Halon 1301 (CF3Br) is used as a total flooding fire suppression gas on Royal Canadian Navy ships and submarines. Because it is an ozone depleting substance it will not be used on new build naval vessels. There are a number of non-ozone depleting gaseous agents that have been evaluated as alternatives for Halon 1301 for use in normally occupied spaces. These include FE-13 (HFC-23 or CHF3), NAF-S111 (HCFC Blend), FM-200 (HFC-227ea or CF3CHFCF3, PFC-410 or CEA 410 (C4F10), and Novec 1230 (1,1,1,2,2,4,5,5,5-nonafluoro-4-trifluoromethylpentan-3-one). A concern associated with all these gaseous agents is the release of acid gases such as hydrogen fluoride (HF) when they are exposed to high temperatures and hot surfaces. The concentrations of an acid gas such as HF will have a direct impact on the risks to firefighters during re-entry procedures. In this report the results of research on the factors affecting the concentration of HF in a space after discharge of Halon 1301 or alternative gaseous agents are reviewed. -

Apple Regulated Substances Specification 069-0135-L
Apple Regulated Substances Specification 069-0135-L Revision ECO # Approver Date Revision Description L 0027247044 JB March 31, 2021 See Section 13 for full revision history. Scope | Definitions | Restricted Substances in Products | Reportable Substances and Future Restrictions in Products | Notifying Apple of Chemical Phase Out and Reformulation from Suppliers | Restrictions in Manufacturing Processes | Reportable Substances and Future Restrictions in Manufacturing Processes | Supplementary Specifications | Demonstrating Compliance | Waiver Process | Full Material Disclosure | Chemical Safety Disclosure | Revision History | Referenced Documents | Appendices 1. Scope It’s Apple’s mission to make sure that anyone who assembles, uses, or recycles an Apple product can do so safely. We have led the industry in removing many harmful substances from our product designs, and we go to great lengths to make sure that they stay that way. We are constantly designing our products to be better for the environment, and for people. This Regulated Substances Specification describes Apple’s global restrictions on the use of certain chemical substances or materials in Apple products, accessories, manufacturing processes, and packaging used for shipping products to Apple’s end-customers. Restrictions are derived from international laws or directives, regulatory agencies, eco-label requirements, environmental standards, and Apple policies. Apple’s restrictions may go beyond regulatory requirements in order to protect human health and the environment. This specification is not an exhaustive list of all chemicals of concern. Apple suppliers should take action to understand the human health and environmental impacts of all chemicals used in the manufacturing process and present in parts and materials supplied to Apple. Suppliers should take action to reduce or eliminate the use of chemicals of concern listed in this specification as a first step, as well as comply with all applicable regulations. -

(12) United States Patent (10) Patent No.: US 6,391,182 B2 Smeltzer Et Al
USOO6391.182B2 (12) United States Patent (10) Patent No.: US 6,391,182 B2 Smeltzer et al. (45) Date of Patent: May 21, 2002 (54) ELECTROCHEMICAL FLUORINATION 5,286.352 A 2/1994 Hansen et al. ............ 204/59 F USING INTERRUPTED CURRENT 5,322,597 A 6/1994 Childs et al. ............. 204/59 F 5,387.323 A 2/1995 Minday et al. ........... 204/59 F (75) Inventors: John C. Smeltzer, Woodbury, MN 5,427,656 A 6/1995 Behr et al. ................ 204/59 F (US); Charles F. Kolpin, River Falls, 5,474,657 A 12/1995 Hansen et al. - - - - - - - - - - - - 204/59 F WI (US) FOREIGN PATENT DOCUMENTS (73) Assignee: 3M Innovative Properties Company, DE 741,399 11/1955 St. Paul, MN (US) DE 785492 10/1957 FR 2 604 189 3/1988 (*) Notice: Subject to any disclaimer, the term of this WO WO 94/13857 6/1994 patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. OTHER PUBLICATIONS AlsmeySmeyer et al.,1. “El ectrochemicahemical FluorinatiuOrnation andd iItS (21) Appl. No.: 09/833,360 Applications,” Organofluorine Chemistry. Principles and (22) Filed: Apr. 12, 2001 Commerical Applications, Chapter 5 (1994) pp. 121-143. O O No Month Available. Related U.S. Application Data W.V. Childs, et al., “Anodic Fluorination' Organic Electro (60) Divisionivision of application No. 09/464,781,föl, filedilled onOn Dec.Jec. 17If, chemistry, H. Lund and M. Bazer eds, Marcel Dekker Inc., application1999, now Pat.No. No. 09/074,830, 6,267,865, filed and aon continuation-in-part May 8, 1998, now of Ney. -

Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2018 – Annex 6
ANNEX 6 Additional Information 6.1. Global Warming Potential Values Global Warming Potential (GWP) is intended as a quantified measure of the globally averaged relative radiative forcing impacts of a particular greenhouse gas. It is defined as the cumulative radiative forcing–both direct and indirect effects⎯ integrated over a specific period of time from the emission of a unit mass of gas relative to some reference gas (IPCC 2007). Carbon dioxide (CO2) was chosen as this reference gas. Direct effects occur when the gas itself is a greenhouse gas. Indirect radiative forcing occurs when chemical transformations involving the original gas produce a gas or gases that are greenhouse gases, or when a gas influences other radiatively important processes such as the atmospheric lifetimes of other gases. The relationship between kilotons (kt) of a gas and million metric tons of CO2 equivalents (MMT CO2 Eq.) can be expressed as follows: MMT MMT CO Eq. = (kt of gas) (GWP) 2 1,000 kt where, MMT CO2 Eq. = Million metric tons of CO2 equivalent kt = kilotons (equivalent to a thousand metric tons) GWP = Global warming potential MMT = Million metric tons GWP values allow policy makers to compare the impacts of emissions and reductions of different gases. According to the IPCC, GWP values typically have an uncertainty of 35 percent, though some GWP values have larger uncertainty than others, especially those in which lifetimes have not yet been ascertained. In the following decision, the countries who are Parties to the United Nations Framework Convention on Climate Change (UNFCCC) have agreed to use consistent GWP values from the IPCC Fourth Assessment Report (AR4), based upon a 100 year time horizon, although other time horizon values are available (see Table A-252). -

Chemical Industry Emissions
Chapter 3: Chemical Industry Emissions CHAPTER 3 CHEMICAL INDUSTRY EMISSIONS 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories 3.1 Volume 3: Industrial Processes and Product Use Authors Sections 3.3 Retno Gumilang Dewi (Indonesia) and María José López Blanco (Spain) Sections 3.10 Karen S. Schaffner (USA), Deborah A. Ottinger (USA) and Brian T. Mader (USA) Sections 3.11 Håkon Frøysa Skullerud (Norway), Katsuhiko Hirose (Japan) and Samir Tantawi (Egypt) Contributing Authors Sections 3.10 Jeffrey B. Coburn (USA) and Gregory M. Watson (USA) Sections 3.11 Richard Chahine (Canada), Shohei Oyama (Japan) and Nigel Brandon (UK) 3.2 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories Chapter 3: Chemical Industry Emissions Contents 3 Chemical Industry Emissions ....................................................................................................................... 3.8 3.1 Introduction ........................................................................................................................................ 3.8 3.2 Ammonia Production .......................................................................................................................... 3.8 3.3 Nitric Acid Production ........................................................................................................................ 3.8 3.3.1 Introduction ................................................................................................................................... -

Survey of Selected Fluorinated Green- House Gases
Survey of selected fluorinated green- house gases Part of the LOUS-review Environmental project No. 1619, 2014 Title: Authors & contributors: Survey of selected fluorinated greenhouse gases Erik Hansen 1, Per Henrik Pedersen2, Frans Møller Christensen 1, Karen Louise Feilberg 1, Marlies Warming1 1 COWI A/S, Denmark 2Danish Technological Institute Published by: The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. 2014 978-87-93283-24-4 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning re- search and development projects within the environmental sector, financed by study grants provided by the Danish Envi- ronmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. While the information provided in this report is believed to be accurate, the Danish Environmental Protection Agency disclaims any responsibility for possible inaccuracies or omissions and consequences that may flow from them. Neither the Danish Environmental Protection Agency nor COWI or any individual involved in the preparation of this publication shall be liable for any injury, loss, damage or prejudice of any kind that may be caused by persons who have acted based on their understanding of the information contained in this publication. Sources must be acknowledged. 2 Survey of selected fluorinated greenhouse gases Contents Preface ........................................................................................................................... 5 Summary and conclusions ........................................................................................... -

Halon Replacement Options for Aircraft”
“Halon Replacement Options for Aircraft” Robert E. Tapscott Center for Global Environmental Technologies University of New Mexico Albuquerque, New Mexico USA Louise C. Speitel Fire Safety Section DOT/FAA/William J. Hughes Technical Center Atlantic City, New Jersey USA International Aircraft Fire and Cabin Safety Research Conference Atlantic City, New Jersey USA, 16-20 November 1998 ABSTRACT In 1987, an international treaty—the Montreal Protocol—was established to control the release of materials that cause stratospheric ozone depletion. Under the Protocol, the production of halon fire and explosion protection agents was phased out in all industrialized countries at the end of 1993. To date, no environmentally acceptable halon replacement equivalent to the existing halons in toxicity, effectiveness, and dimensionality across all applications has been identified. A large number of new agents and technologies that provide adequate protection in most applications (usually, with tradeoffs) have, however, been developed. This paper presents an overview of halon options that have been commercialized or are near to commercialization. The term “options” is used here for anything that could be used in place of halons. There are two types of options: (1) “replacements” are halocarbon agents chemically similar to the present halons; (2) “alternatives,” are everything else. “Chemical alternatives” are materials such as carbon dioxide, foam, water, and dry chemical, which are chemically distinct from the halons. “Engineering alternatives” (not covered here) refer to approaches such as rapid response and fire resistant structures. HALON FIRE EXTINGUISHANTS Halon fire extinguishing agents are low boiling point halocarbons (chemical compounds that contain carbon and one or more of the halogen elements—fluorine, chlorine, bromine, and iodine) that have been extensively used in the past to suppress fires and to protect against explosions (Table 1). -

DOT/FAA/AR-99/63 Options to the Use of Halons For
DOT/FAA/AR-99/63 Options to the Use of Halons for Office of Aviation Research Aircraft Fire Suppression Washington, D.C. 20591 Systems—2002 Update February 2002 Final Report This document is available to the U.S. public through the National Technical Information Service (NTIS), Springfield, Virginia 22161. U.S. Department of Transportation Federal Aviation Administration NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents or use thereof. The United States Government does not endorse products or manufacturers. Trade or manufacturer's names appear herein solely because they are considered essential to the objective of this report. This document does not constitute FAA certification policy. Consult your local FAA aircraft certification office as to its use. The following commercial products (requiring trademark ® or ) are mentioned in this report. Because of the frequency of usage, trademarks are not indicated. Mention of a product does not constitute endorsement or rejection of the product. Argonite FE-232 Inergen AeroGen FE-13 Iodoguard Aero-K FE-227 KD-A 96 AquaMist FE-241 MicroDrop AquaSafe Firefox Micro-k Argotec Firepak MicroMist BLITZ Firescope Mistex CEA-308 Fire Protection Handbook Monnex CEA 410 Fire-X-Plus NAF P-III CEA 614 FlameOut NAF S-III Cease Fire FogJet NN100 Chemetron FOGTEC PyroGen ColdFire FM-200 Pyrozone ColdFire 302 FS 0140 Sem-Safe Envirogel Halonyzer S.F.E. FEAS Halotron I Soyus FE-25 Hi-fog Statham FE-36 IAI Watermist Ultra Fog This report is available at the Federal Aviation Administration William J.