DOT/FAA/AR-99/63 Options to the Use of Halons For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Status of Industry Efforts to Replace Halon Fire Extinguishing Agents
STATUS OF INDUSTRY EFFORTS TO REPLACE HALON FIRE EXTINGUISHING AGENTS Robert T. Wickham, P.E. March 16, 2002 WICKHAM ASSOCIATES NOTICE This document is disseminated under the sponsorship of the U.S. Environmental Protection Agency in the interest of information exchange. The views expressed in this report are those of the author and do not necessarily reflect those of the US Environmental Protection Agency. The US Environmental Protection Agency does not assume liability for the contents or use thereof and further the Agency does not endorse products or manufacturers. Trade or manufacturer's names appear herein solely because they are considered essential to the objective of this report. The following commercial products (requiring a trademark designation ® or ™) are mentioned in this report. Due to the frequency of usage, trademarks are not indicated. Mention of a product does not constitute endorsement or rejection of the product. Ansul FE-25 Argonite FE-36 Argotec FM-200 CEA-410 Halotron I CEA-614 Inergen CEA-308 NAF P-IV Envirogel NAF S-III FE-13 NN100 FE-227 Triodide FE-241 There are no restrictions on copying or distribution of this document. Additional copies of this report are available from ….. Wickham Associates 9 Winding Brook Drive Stratham, New Hampshire 03885 USA Tel: +603-772-3229 Fax: +603-772-7305 email: [email protected] The report is available at http://home.attbi.com/~wickham/downloads.htm for downloading in Adobe Acrobat portable document format (pdf). Preface This report is not intended to be a market study, but instead a snapshot of the progress industry and the government are making in employing non ozone depleting alternatives to halons in the fire protection sector. -
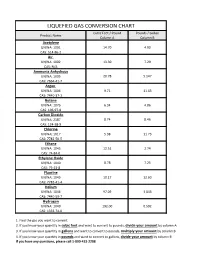
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -
Ozone Depleting Substances
Ozone Depleting Substances What are they? Ozone depleting substances are chemicals that destroy the earth’s protective ozone layer. They include: • chlorofluorocarbons (CFCs) • halons • carbon tetrachloride (CCl4) • methyl chloroform (CH3CCl3) • hydrobromofluorocarbons (HBFCs) • hydrochlorofluorocarbons (HCFCs) • methyl bromide (CH3Br) • bromochloromethane (CH2BrCl) The use of these chemicals is controlled by the Montreal Protocol on Substances that Deplete the Ozone Layer (the Montreal Protocol). There are other ozone depleting substances, but their ozone depleting effects are very small, so they are not controlled by the Montreal Protocol. One kilogram of halon 1211 can destroy 50 tonnes of ozone What is ozone depleting potential? Ozone depleting potential is a measure of how much damage a chemical can cause to the ozone layer compared with a similar mass What did we use ozone depleting of trichlorofluoromethane (CFC-11). CFC-11, with an ozone depleting potential of 1.0, is substances for? used as the base figure for measuring ozone depletion potential. The higher the number, The main uses of ozone depleting substances the more damage a chemical can cause to include; CFCs and HCFCs in refrigerators and the ozone layer. Bromotrifluoromethane air conditioners, HCFCs and halons in fire (halon -1301) has an ozone depleting potential extinguishers, CFCs and HCFCs in foam, CFCs of 10.0. Carbon dioxide (CO2) is a naturally and HCFCs as aerosol propellants and methyl occurring greenhouse gas, but has an ozone bromide for fumigation of soil, structures and depleting potential of 0. goods to be imported or exported. Have we stopped using ozone Why do we still use some ozone depleting substances? depleting substances? Production of most ozone depleting Some substances with a high ozone depleting substances has been phased out under the potential are still used in quarantine and Montreal Protocol. -

Prepared by 3M
DRAFT ECOTOXICOLOGY AND ENVIRONMENTAL FATE TESTING OF SHORT CHAIN PERFLUOROALKYL COMPOUNDS RELATED TO 3M CHEMISTRIES XXX XX, 2008 Prepared by 3M Page 1 CONFIDENTIAL - SUBJECT TO A PROTECTIVE ORDER ENTERED IN 3M MN01537089 HENNEPIN COUNTY DISTRICT COURT, NO. 27-CV-10-28862 2231.0001 DRAFT TABLE OF CONTENTS Page Introduction (describes propose, goals, document organization .... ) 3 1) Degradation modes 4-27 Basic degradation pathways, Conversion routes from polymer to monomer & degradants or residual intermediates to degradants) 2) Degradants & Intermediates - Phys. Chem properties and test data by section & chapter i) Fluorinated Sulfonic acids and derivatives a) PFBS (PBSK) 28-42 b) PFBSI 43-44 ii) Fluorinated Carboxylates a) TFA 46-55 b) PFPA 56-59 c) PFBA (APFB) 60-65 d) MeFBSAA (MeFBSE Acid, M370) 66-68 iii) Fluorinated Non-ionics a) MeFBSE 70-74 b) MeFBSA 75-80 c) FBSE 81-84 d) FBSA 85-88 e) HxFBSA 89-91 iv) Fluorinated Inerts & volatiles a) PBSF 94-97 b) NFB, C4 hydride, CF3CF2CF2CF2-H 98-99 3) Assessments X Glossary 100-102 References/Endnotes 105- Page 2 CONFIDENTIAL - SUBJECT TO A PROTECTIVE ORDER ENTERED IN 3M MN01537090 HENNEPIN COUNTY DISTRICT COURT, NO. 27-CV-10-28862 2231.0002 DRAFT Introduction to Environmental White Papers on Perfluoroalkyl acids related to 3M chemistries Perfluorochemicals have been commonplace in chemical industry over 50 years but until recently there has been little information on environmental fate and effects avialble in open literature. The following chapters summarize the findings of"list specific C4 intermediates PFBS, PFBSI, PFBA, PFPA, MeFBSAA, TFA, MeFBSE, FBSA, HxFBSA, FBSE, PBSF, NFB " As background, 3M announced on May 16, 2000 the voluntary manufacturing phase out of perfluorooctanyl chemicals which included perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), and PFOS-related chemistries. -

Alkyl and Fluoroalkyl Manganese Pentacarbonyl Complexes As
En vue de l'obtention du DOCTORAT DE L'UNIVERSITÉ DE TOULOUSE Délivré par : Institut National Polytechnique de Toulouse (Toulouse INP) Discipline ou spécialité : Chimie Organométallique et de Coordination Présentée et soutenue par : M. ROBERTO MORALES CERRADA le jeudi 15 novembre 2018 Titre : Complexes de manganèse pentacarbonyle alkyle et fluoroalkyle comme modèles d'espèces dormantes de l'OMRP Ecole doctorale : Sciences de la Matière (SDM) Unité de recherche : Laboratoire de Chimie de Coordination (L.C.C.) Directeur(s) de Thèse : MME FLORENCE GAYET M. BRUNO AMEDURI Rapporteurs : M. GERARD JAOUEN, UNIVERSITE PARIS 6 Mme SOPHIE GUILLAUME, CNRS Membre(s) du jury : M. MATHIAS DESTARAC, UNIVERSITE TOULOUSE 3, Président M. BRUNO AMEDURI, CNRS, Membre M. HENRI CRAMAIL, INP BORDEAUX, Membre Mme FLORENCE GAYET, INP TOULOUSE, Membre A mi abuelo Antonio ‐ i ‐ ‐ ii ‐ Remerciements Ce travail a été réalisé dans deux unités de recherche du CNRS : le laboratoire de Chimie de Coordination (LCC) à Toulouse, au sein de l’équipe LAC2, et l’Institut Charles Gerhardt de Montpellier (ICGM), au sein de l’équipe IAM. Il a été codirigé par Dr. Florence Gayet et Dr. Bruno Améduri. Je tiens tout d’abord à remercier Dr. Azzedine Bousseksou, directeur du LCC, et Dr. Patrick Lacroix‐Desmazes, directeur de l’équipe IAM à l’ICGM, pour avoir accepté de m’accueillir au sein de ses laboratoires. Je remercie tout particulièrement mes directeurs de thèse, Dr. Florence Gayet et Dr. Bruno Améduri, pour m’avoir encadré durant ces trois années de doctorat. Un immense merci à tous les deux pour tous leurs conseils, leur patience et leurs connaissances qui m’ont apporté et qui m’ont permis de mener à bien ce travail. -

Halons Technical Options Committee 2018 AssessMent Report
Halons Technical Options Committee 2018 Assess ment Report Volume 1 Montreal Protocol on Substances that Deplete the Ozone Layer Ozone Secretariat MONTREAL PROTOCOL ON SUBSTANCES THAT DEPLETE THE OZONE LAYER REPORT OF THE HALONS TECHNICAL OPTIONS COMMITTEE DECEMBER 2018 VOLUME 1 2018 ASSESSMENT REPORT i Montreal Protocol on Substances that Deplete the Ozone Layer Report of the Halons Technical Options Committee December 2018 Volume 1 2018 ASSESSMENT REPORT The text of this report is composed in Times New Roman Co-ordination: Halons Technical Options Committee Composition of the report: Halons Technical Options Committee Reproduction: Ozone Secretariat Date: December 2018 Under certain conditions, printed copies of this report are available from: UNITED NATIONS ENVIRONMENT PROGRAMME Ozone Secretariat, P.O. Box 30552, Nairobi, Kenya This document is also available in portable document format from the Ozone Secretariat's website: https://ozone.unep.org/sites/default/files/Assessment_Panel/Assessment_Panels/TEAP/R eports/HTOC/HTOC_assessment_2018.pdf No copyright involved. This publication may be freely copied, abstracted and cited, with acknowledgement of the source of the material. ISBN: 978-9966-076-48-9 iii Disclaimer The United Nations Environment Programme (UNEP), the Technology and Economic Assessment Panel (TEAP) Co-chairs and members, the Technical Options Committees Co-chairs and members, the TEAP Task Forces Co-chairs and members, and the companies and organisations that employ them do not endorse the performance, worker safety, or environmental acceptability of any of the technical options discussed. Every industrial operation requires consideration of worker safety and proper disposal of contaminants and waste products. Moreover, as work continues - including additional toxicity evaluation - more information on health, environmental and safety effects of alternatives and replacements will become available for use in selecting among the options discussed in this document. -

Trifluoroiodomethane As an Environmentally Friendly Gas for Water Patterning by Plasma Etching Process
Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. Please Note: The author retains the copyright while the New Jersey Institute of Technology reserves the right to distribute this thesis or dissertation Printing note: If you do not wish to print this page, then select “Pages from: first page # to: last page #” on the print dialog screen The Van Houten library has removed some of the personal information and all signatures from the approval page and biographical sketches of theses and dissertations in order to protect the identity of NJIT graduates and faculty. ABSTRACT TRIFLUOROIODOMETHANE AS AN ENVIRONMENTALLY FRIENDLY GAS FOR WAFER PATTERNING BY PLASMA ETCHING PROCESS by Krit Aryusook Trifluoroiodomethane (CF3I), a non-global warming gas, has been investigated with study as a substitute for typical CFC etchants, such as CF,, and C2F6„ used in wafer pattering technology. This investigation was carried out by exposing dielectric films of silicon oxide (SiO2) and silicon nitride (Si3N4) in CF3I and C2 F6/02 (used as a reference) plasma environments. -

ACE Tips for Filing EPA ODS
Automated Commercial Environment ACE Tips for Filing EPA ODS June, 2018 Ozone-depleting substances (ODS) deplete the stratospheric ozone layer when the chlorine and bromine atoms that they contain come into contact with ozone molecules. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. ODS that release chlorine include chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform. ODS that release bromine include halons and methyl bromide. HTS code Ozone-Depleting Substance 2903.14.0000 Carbon tetrachloride What commodities 2903.19.6010 Methylchloroform (1,1,1-Trichloroethane) are ODS used in? 2903.39.1520 Methyl bromide 2903.71.0000 Chlorodifluoromethane (HCFC-22) ODS have been used 2903.72.0020 Dichlorotrifluoroethane (HCFC-123) for refrigeration, air 2903.73.0000 Dichlorofluoroethanes (HCFC-141, 141b) conditioning, insulation, 2903.74.0000 Chlorodifluoroethanes (HCFC-142, 142b) solvents, aerosol 2903.75.0000 Dichloropentafluoropropanes propellants, and in other (HCFC-225, 225ca, 225cb) sectors. Currently, the United 2903.76.0010 Bromotrifluoromethane (Halon 1301) States is in the 2903.76.0050 Bromochlorodifluoromethane (Halon 1211), process of phasing bromotrifluoromethane (Halon 1301), and out HCFCs, but in the dibromotetrafluoroethanes (Halon 2402), Other interim limited imports are 2903.77.0010 Trichlorofluoromethane (CFC-11) allowed. CFCs, 2903.77.0020 Trichlorotrifluoroethanes (CFC-113, CFC-113a) methyl bromide, halon, 2903.77.0030 -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

1 HUMAN HEALTH SAFETY EVALUATION of HALON REPLACEMENT CANDIDATES Darol E. Dodd1, Gary W. Jepson1, and Joseph A. Macko, Jr.2 1Air
HUMAN HEALTH SAFETY EVALUATION OF HALON REPLACEMENT CANDIDATES Darol E. Dodd1, Gary W. Jepson1, and Joseph A. Macko, Jr.2 1Air Force Research Laboratory/Operational Toxicology Branch (AFRL/HEST) – ManTech Environmental Technology, Inc. Wright-Patterson Air Force Base, Ohio 2Toxicology Directorate (MCHB-TS-T)/U.S. Army Center for Health Promotion & Preventive Medicine Aberdeen Proving Ground, Maryland Next-Generation Fire Suppression Technology Program (NGP) Project 3B/1/89 Final Report – Section II of II This research is part of the Department of Defense’s Next-Generation Fire Suppression Technology Program, funded by the DoD Strategic Environmental Research and Development Program (SERDP) 1 TABLE OF CONTENTS Page INTRODUCTION……………………………...………………………………………………….4 PHASE 1 – TOXICITY SCREENING METHODS……………………...……………………….5 Chemical/physical properties……………………………………………….…………….6 Existing toxicity literature…………………………………………………..…………….6 Preliminary “specific use” scenarios………………………………………..…………….7 Qualitative/quantitative structure activity relationships……………………..……………7 In vitro screens……………………………………………………………...…………….8 Acute irritation tests………………………………………………………………………9 Acute toxicity tests…………………………………………………………..…………..10 The “limit test”…………………………………………………………………..11 The “specific use” scenario test……………………………………..…………..12 Genotoxicity tests (Phase 1)………………………………………………..……………13 DECISION POINT 1……………………………………………………………………14 Concentration x time (C x T) relationships……………………….……………18 Stop or continue testing…………………………………………..…………….18 PHASE 2 – TOXICITY -

Cf3i) for Application in Gas-Insulated Lines
INVESTIGATION ON THE FEASIBILITY OF TRIFLUOROIODOMETHANE (CF3I) FOR APPLICATION IN GAS-INSULATED LINES Lujia Chen Advanced High Voltage Engineering Research Centre Cardiff University A thesis submitted for the degree of Doctor of Philosophy July, 2015 DECLARATION This work has not been submitted in substance for any other degree or award at this or any other university or place of learning, nor is being submitted concurrently in candidature for any degree or other award. Signed ………………………………………… (Candidate) Date ………………………… STATEMENT 1 This thesis is being submitted in partial fulfilment of the requirements for the degree of PhD. Signed ………………………………………… (Candidate) Date ………………………… STATEMENT 2 This thesis is the result of my own independent work/investigation, except where otherwise stated. Other sources are acknowledged by explicit references. The views expressed are my own. Signed ………………………………………… (Candidate) Date ………………………… STATEMENT 3 I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter-library loan, and for the title and summary to be made available to outside organisations. Signed ………………………………………… (Candidate) Date ………………………… i ACKNOWLEDGEMENTS I would like to express my deepest gratitude to my supervisors, Professors Abderrahmane Haddad and Huw Griffiths, for their patient guidance and encouragement. My sincere thanks also go to Professor Ronald Thomas Waters for his invaluable knowledge and expertise in the field of gas discharge. In addition, I would also like to thank the Engineering and Physical Sciences Research Council (EPSRC) for funding this PhD studentship, which financially supports me to concentrate on my academic research. For the experimental work, I greatly appreciate the timely help from those people. Thank you to Professors Kunihiko Hidaka and Akiko Kumada and to everyone from the high voltage research group of Tokyo University, Japan, for the fruitful discussions and for making my research visit there thoroughly enjoyable. -

This Table Gives the Standard State Chemical Thermodynamic Properties of About 2500 Individual Substances in the Crystalline, Liquid, and Gaseous States
STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES This table gives the standard state chemical thermodynamic properties of about 2500 individual substances in the crystalline, liquid, and gaseous states. Substances are listed by molecular formula in a modified Hill order; all substances not containing carbon appear first, followed by those that contain carbon. The properties tabulated are: DfH° Standard molar enthalpy (heat) of formation at 298.15 K in kJ/mol DfG° Standard molar Gibbs energy of formation at 298.15 K in kJ/mol S° Standard molar entropy at 298.15 K in J/mol K Cp Molar heat capacity at constant pressure at 298.15 K in J/mol K The standard state pressure is 100 kPa (1 bar). The standard states are defined for different phases by: • The standard state of a pure gaseous substance is that of the substance as a (hypothetical) ideal gas at the standard state pressure. • The standard state of a pure liquid substance is that of the liquid under the standard state pressure. • The standard state of a pure crystalline substance is that of the crystalline substance under the standard state pressure. An entry of 0.0 for DfH° for an element indicates the reference state of that element. See References 1 and 2 for further information on reference states. A blank means no value is available. The data are derived from the sources listed in the references, from other papers appearing in the Journal of Physical and Chemical Reference Data, and from the primary research literature. We are indebted to M. V. Korobov for providing data on fullerene compounds.