Physical and Chemical Data*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

The Following Carcinogenic Essential Oils Should Not Be Used In
Aromatherapy Undiluted- Safety and Ethics Copyright © Tony Burfield and Sylla Sheppard-Hanger (2005) [modified from a previous article “A Brief Safety Guidance on Essential Oils” written for IFA, Sept 2004]. Intro In the last 20 years aromatherapy has spread its influence to the household, toiletries and personal care areas: consumer products claiming to relax or invigorate our psyche’s have invaded our bathrooms, kitchen and living room areas. The numbers of therapists using essential oils in Europe and the USA has grown from a handful in the early 1980’s to thousands now worldwide. We have had time to add to our bank of knowledge on essential oils from reflecting on many decades of aromatherapeutic development and history, the collection of anecdotal information from practicing therapists, as well as from clinical & scientific investigations. We have also had enough time to consider the risks in employing essential oils in therapy. In the last twenty years, many more people have had accidents, been ‘burnt’, developed rashes, become allergic, and become sensitized to our beloved tools. Why is this? In this paper, we hope to shed light on this issue, clarify current safety findings, and discuss how Aromatherapists and those in the aromatherapy trade (suppliers, spas, etc.) can interpret this data for continued safe practice. After a refresher on current safety issues including carcinogenic and toxic oils, irritant and photo-toxic oils, we will look at allergens, oils without formal testing, pregnancy issues and medication interactions. We will address the increasing numbers of cases of sensitization and the effect of diluting essential oils. -

Anti-Eczema Mechanism of Action of Nigella Sativa for Atopic Dermatitis: Computer-Aided Prediction and Pathway Analysis Based on Protein-Chemical Interaction Networks
68 BIOMOLECULAR AND HEALTH SCIENCE JOURNAL 2019 OCTOBER, VOL 02 (02) ORIGINAL ARTICLE Anti-eczema Mechanism of Action of Nigella sativa for Atopic Dermatitis: Computer-aided Prediction and Pathway Analysis Based on Protein-chemical Interaction Networks Meidyta Sinantryana Widyaswari1, Iis Noventi2, Herdiantri Sufriyana3,4* 1Department of Dermatology and Venereology, College of Medicine, University of Nahdlatul Ulama Surabaya, Indonesia 2Department of Medical-Surgical Nursing, College of Nursing and Midwifery, University of Nahdlatul Ulama Surabaya, Indonesia 3Department of Medical Physiology, College of Medicine, University of Nahdlatul Ulama Surabaya, Surabaya, Indonesia 4Graduate Institute of Biomedical Informatics, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan A R T I C L E I N F O A B S T R A C T Article history: Introduction: Black cumin (Nigella sativa) is widely used to treat various diseases. It is also Received 26 August 2019 believed to relief skin conditions accompanied by itching symptom, such as atopic dermatitis Received in revised form 14 (AD) or eczema. However, the anti-eczema mechanism of action is still unclear. The aims of this October 2019 syudy was to identify anti-eczema mechanism of action of N. sativa for AD using computer aided Accepted 21 October 2019 prediction and pathway analysis based on protein-chemical networks. Available online 31 October 2019 Methods: We utilized dataset consisting chemical compounds of N. sativa from KNApSAcK. It is a comprehensive species-metabolite relationship database. Using canonical SMILES strings that Keywords: encode molecular structures of each compound, we predicted the probabilities of activity (Pa) for Nigella sativa, anti-eczema effect based on PASS algorithms. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Mercury--Quicksilver
scueu« No. 12 Mineral Technology Series No 6 University of Arizona Bulletin Mercury---Quicksilver By P. E. JOSEPH SECOND ISSUE NOVEMBER, 1916. Entered as second class matter November 2:1, 191~, at the postoftice at Tucson, Arizona. under the Act ot August 24, 1912. Issued weekb". September to Ya)·. PUBLISHED BY THE University of Arizona Bureau of Mines CHARLES F. WILLIS, Director TUCSON, ARIZONA 1916-17 BIBLIOGRAPHY Bancroft, Howland. Notes on the occurrence of cinnabar in central western Arizona. U. S. G. S. Bull. 430, pp. 151-153, 1910. Becker, G. F. Geology of- the quicksilver deposits of the Pacific slope, with atlas. Mon. 13, p. 486, 1888. Only the atlas in stock. Quicksilver Ore Deposits; Mineral Resources U. S. for 1892, pp. 139-168, 1893. Christy, S. B. Quicksilver reduction at New Almaden, Cal. Min- eral Resources U. S. for 1883-1884, pp. 603-636, 1885. Hillebrand, W. F., and Schaller, W. T. Mercury miner-als from Terlingua, Tex. U. S. G. S. Bull. 405, pp. 174, 1909. McCaskey, H. D. Quicksilver in 1912; Mineral Resources U. S. for 1912, Pt. 1, pp. 931-948, 1913. Quicksilver in 1913-Production and Resources; Mineral Resources U. S. for 1913, Pt. 1, pp. 197-212, 1914. Melville, W. H., and Lindgren, Waldemar. Contributions to the mineralogy of the Pacific coast. U. S. G. S. Bull. 61, 30 pp., 1890. Parker, E. W. Quicksilver; Twenty-first Ann. Rept. U. S. G. S., Pt. 6, pp. 273-283, 1901. University of Arizona Bulletin BULLETIN No. 12 SECOND ISSUE, NOVEMBER, 1916 MERCURY-QUICKSILVER By P. -

WO 2015/061786 A2 30 April 2015 (30.04.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/061786 A2 30 April 2015 (30.04.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every E21B 43/26 (2006.01) E21B 47/06 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 14/062440 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 27 October 2014 (27.10.2014) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/895,873 25 October 2013 (25. 10.2013) US (84) Designated States (unless otherwise indicated, for every 61/898, 107 31 October 2013 (3 1. 10.2013) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (72) Inventors; and TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicants : CONWAY, Andrew, Bryce [US/US]; 1501 TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, Timbercreek Drive, Weatherford, OK 73096 (US). -

Rational Chemical Design of Solar-Powered Nano Photocatalysts for Environmental Applications
Rational Chemical Design of Solar-Powered Nano Photocatalysts for Environmental Applications Being a Thesis submitted for the Degree of Doctor of Philosophy degree (PhD) in the University of Hull By Khadijah Mohammedsaleh M Katubi (January 2015) Declaration I, Khadijah Mohammedsaleh M Katubi of Student Number: 200892573, hereby declare that this project, Rational Chemical Design of Solar-Powered Nano Photocatalysts for Environmental Applications being the requirement of the Hull University PhD Faculty of Science of Engineering (FOSE), Chemistry department, Academic Year 2015, is entirely of my own effort and work with the exception of excerpts cited from other works of which the sources were duly noted and acknowledged in the bibliography. Copyright © 2015 by Khadijah Mohammedsaleh M Katubi. All rights reserved. No part of this publication may be reproduced without the written permission of the copyright holder E-mail: [email protected] and [email protected] Acknowledgements Firstly, I would like to express my deepest gratitude to Almighty Allah for the assistance. Allah has given me patience, continued support and guidance without which this work would not be accomplished. Secondly, I owe sincere thanks to my supervisor Dr M. Grazia Francesconi for her continued backing, sincere affection and the assistance given to me throughout my PhD. I would also like to thank Dr Nigel A. Young for his assistance in FT-IR and UV-Vis spectroscopy. Lots of thanks go to Dr M. Grazia Francesconi, Dr Timothy J. Prior, Dr Vincent Rocher and PhD Simon Fellows for the assistance and sharing knowledge in XRD analysis. Many thanks go to Zabeada Aslamb at Leeds University for their help in TEM, SAED and EELS. -

Vapor-Liquid Equilibrium Measurements of the Binary Mixtures Nitrogen + Acetone and Oxygen + Acetone
Vapor-liquid equilibrium measurements of the binary mixtures nitrogen + acetone and oxygen + acetone Thorsten Windmann1, Andreas K¨oster1, Jadran Vrabec∗1 1 Lehrstuhl f¨urThermodynamik und Energietechnik, Universit¨atPaderborn, Warburger Straße 100, 33098 Paderborn, Germany Abstract Vapor-liquid equilibrium data of the binary mixtures nitrogen + acetone and of oxygen + acetone are measured and compared to available experimental data from the literature. The saturated liquid line is determined for both systems at specified temperature and liquid phase composition in a high-pressure view cell with a synthetic method. For nitrogen + acetone, eight isotherms between 223 and 400 K up to a pressure of 12 MPa are measured. For oxygen + acetone, two isotherms at 253 and 283 K up to a pressure of 0.75 MPa are measured. Thereby, the maximum content of the gaseous component in the saturated liquid phase is 0.06 mol/mol (nitrogen) and 0.006 mol/mol (oxygen), respectively. Based on this data, the Henry's law constant is calculated. In addition, the saturated vapor line of nitrogen + acetone is studied at specified temperature and pressure with an analytical method. Three isotherms between 303 and 343 K up to a pressure of 1.8 MPa are measured. All present data are compared to the available experimental data. Finally, the Peng-Robinson equation of state with the quadratic mixing rule and the Huron-Vidal mixing rule is adjusted to the present experimental data for both systems. Keywords: Henry's law constant; gas solubility; vapor-liquid equilibrium; acetone; nitrogen; oxygen ∗corresponding author, tel.: +49-5251/60-2421, fax: +49-5251/60-3522, email: [email protected] 1 1 Introduction The present work is motivated by a cooperation with the Collaborative Research Center Tran- sregio 75 "Droplet Dynamics Under Extreme Ambient Conditions" (SFB-TRR75),1 which is funded by the Deutsche Forschungsgemeinschaft (DFG). -
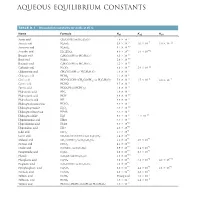
Aqueous Equilibrium Constants
BLB_APP_D_1062-1063hr.qxp 11/8/10 2:27 PM Page 1062 APPENDIX D AQUEOUS EQUILIBRIUM CONSTANTS TABLE D.1 • Dissociation Constants for Acids at 25 ˚C Name Formula Ka1 Ka2 Ka3 -5 Acetic acid CH3COOH (or HC2H3O2) 1.8 * 10 * -3 * -7 * -12 Arsenic acid H3AsO4 5.6 10 1.0 10 3.0 10 * -10 Arsenous acid H3AsO3 5.1 10 * -5 * -12 Ascorbic acid H2C6H6O6 8.0 10 1.6 10 * -5 Benzoic acid C6H5COOH (or HC7H5O2) 6.3 10 * -10 Boric acid H3BO3 5.8 10 * -5 Butanoic acid C3H7COOH (or HC4H7O2) 1.5 10 * -7 * -11 Carbonic acid H2CO3 4.3 10 5.6 10 * -3 Chloroacetic acid CH2ClCOOH (or HC2H2O2Cl) 1.4 10 * -2 Chlorous acid HClO2 1.1 10 * -4 * -5 -7 Citric acid HOOCC(OH) (CH2COOH)2 (or H3C6H5O7) 7.4 10 1.7 10 4.0 * 10 - Cyanic acid HCNO 3.5 * 10 4 * -4 Formic acid HCOOH (or HCHO2) 1.8 10 * -5 Hydroazoic acid HN3 1.9 10 - Hydrocyanic acid HCN 4.9 * 10 10 - Hydrofluoric acid HF 6.8 * 10 4 - -7 Hydrogen chromate ion HCrO4 3.0 * 10 * -12 Hydrogen peroxide H2O2 2.4 10 - -2 Hydrogen selenate ion HSeO4 2.2 * 10 * -8 * -19 Hydrogen sulfide H2S 9.5 10 1 10 - Hypobromous acid HBrO 2.5 * 10 9 - Hypochlorous acid HClO 3.0 * 10 8 - Hypoiodous acid HIO 2.3 * 10 11 * -1 Iodic acid HIO3 1.7 10 * -4 Lactic acid CH3CH(OH)COOH (or HC3H5O3) 1.4 10 * -3 * -6 Malonic acid CH2(COOH)2 (or H2C3H2O4) 1.5 10 2.0 10 * -4 Nitrous acid HNO2 4.5 10 * -2 * -5 Oxalic acid (COOH)2 (or H2C2O4) 5.9 10 6.4 10 * -2 * -9 Paraperiodic acid H5IO6 2.8 10 5.3 10 * -10 Phenol C6H5OH (or HC6H5O) 1.3 10 * -3 * -8 * -13 Phosphoric acid H3PO4 7.5 10 6.2 10 4.2 10 * -5 Propionic acid C2H5COOH (or HC3H5O2) 1.3 10 * -

A (Sixth) List of New Mineral Names
352 A (sixth) list of new mineral names: By L. J. Srv.Nc~a, M.A., F.G.S. Assistant in the Mineral Department of the British Museum. [Communicated March 11, 1918.] Achla,~ite. (R. Koechlin, l~iineralogisches Taschenbuch der Wiener Mineralogischen Gesellschaft, 1911, pp. 12, 62 ; Min. Petr. Mitt., 1912, vol. xxxi, p. 91 (Achiardit).) Synonym of Dachiardite (G. D'Achiardi, 1906; 4th list). Aohlusite. W.F. Petterd, 1910. Catalogue of the Minerals of Tasmania, 8rd edit., Hobart, 1910, p. 191; Papers Roy. Soc. Tasmania, 1910, p. 191. A green alteration product of topaz resembling steatite in appearance, but near soda-mica in composition. Derivation not stated, but no doubt from ~X~.J~, mist, alluding to the cloudy alteration of the clear topaz. Aomite-augite. F. Zambonini, 1910. Mineralogia Vesuviana. Mere. Accad. Sci. Fis. Mat. Napoli, vol. xiv, pp. 158, 155 (acmlteaugite). The same as aegirine-augite (H. Rosenbusch, 1902 ; 2nd List), but brown in colour. Aegerite. (~Jineral Resources U.S. Geol. Survey, for 1910, 1911, part ii, p. 886.) Trade-name for a bitumen allied to elaterite. Aconite. (Mineral Resources U.S. Geol. Survey, for 1909, 1911, part ii, p. 738.) Trade-name for a bitumen very similar to elaterite. Albanite. C. I. Istrati and M. A. Mihailescu, 1912. Bul. Soc. Rem~ne ,Sti., vol. xx, p. 626. A bituminous material from Alt~nia. Alleharite. B. Je~.ek, 1912. Zeits. Kryst. Min., vol. li, p. 275 (Alleharit). Small, acicular, orthorhombic crystals, resembling stibnite in appearance, found with vrbaite (q.v.) on specimens of realgar and i Previous lists of this series have been given at the ends of vols. -

Field Performance Testing of Centrifugal Compressors
FIELD PERFORMANCE TESTING OF CENTRIFUGAL COMPRESSORS by Roy B. Pais and Gerald J. Jacobik Dresser Industries, Inc. Dresser Clark Division Olean, New York Roy B. Pais is a Head Product Engi (flow to speed ratio) . Techniques for varying the � ratio neer at the Clark Division of Dresser for both fixed and variable speed drives are discussed. Industries, Inc., Olean, N.Y., where he has worked since 1970. He has A method of calculating horsepower consumed and the responsibility for the design and de capacity of the compressor is shown. Limitations of a field velopment of centrifugal compressors performance test are also commented upon. as well as associated instrumentation and control systems. Field Performance testing of Centrifugal compressors He graduated from the University with meaningful results is an area of considerable interest of Bombay with a B.S. in Mechanical to the process and gas industry. Although much time and Engineering in 1968. In 1970 he ob money are spent on a field test, one must remember that tained an M.S. in Mechanical Engineering from the State the accuracy of the results is not always in proportion to University of New York at Buffalo. the amount of effort put into the test. The preferred meth He is an active member of the A.S.M.E. and is currently od, of course, is to perform the test in the Manufacturer's Chairman of the Olean Section. Shop where facilities are designed for accurate data ac quisition, and subsequent performance computations. Gerald].]acobik is the Test Super· visor of the Dresser-Clark Division Proper planning is essential for conducting a meaning· Test Department, Dresser Industries, ful test. -

PHYSICAL PROPERTIES User's Guide
PHYSICAL PROPERTIES User’s Guide LICENSE AGREEMENT LICENSOR: Chemstations Inc. 2901 Wilcrest Drive, Suite 305 Houston, Texas 77042 U.S.A. ACCEPTANCE OF TERMS OF AGREEMENT BY THE USER YOU SHOULD CAREFULLY READ THE FOLLOWING TERMS AND CONDITIONS BEFORE USING THIS PACKAGE. USING THIS PACKAGE INDICATES YOUR ACCEPTANCE OF THESE TERMS AND CONDITIONS. The enclosed proprietary encoded materials, hereinafter referred to as the Licensed Program(s), are the property of Chemstations Inc. and are provided to you under the terms and conditions of this License Agreement. Included with some Chemstations Inc. Licensed Programs are copyrighted materials owned by the Microsoft Corporation, Rainbow Technologies Inc., and InstallShield Software Corporation. Where such materials are included, they are licensed by Microsoft Corporation, Rainbow Technologies Inc., and InstallShield Software Corporation to you under this License Agreement. You assume responsibility for the selection of the appropriate Licensed Program(s) to achieve the intended results, and for the installation, use and results obtained from the selected Licensed Program(s). LICENSE GRANT In return for the payment of the license fee associated with the acquisition of the Licensed Program(s) from Chemstations Inc., Chemstations Inc. hereby grants you the following non-exclusive rights with regard to the Licensed Program(s): Use of the Licensed Program(s) on more than one machine. Under no circumstance is the Licensed Program to be executed without either a Chemstations Inc. dongle (hardware key) or system authorization code. You agree to reproduce and include the copyright notice as it appears on the Licensed Program(s) on any copy, modification or merged portion of the Licensed Program(s).