Mapping of Fiber Orientation in Human Internal Capsule by Means Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
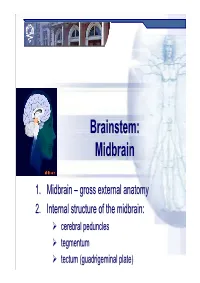
Brainstem: Midbrainmidbrain
Brainstem:Brainstem: MidbrainMidbrain 1.1. MidbrainMidbrain –– grossgross externalexternal anatomyanatomy 2.2. InternalInternal structurestructure ofof thethe midbrain:midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain MidbrainMidbrain –– generalgeneral featuresfeatures location – between forebrain and hindbrain the smallest region of the brainstem – 6-7g the shortest brainstem segment ~ 2 cm long least differentiated brainstem division human midbrain is archipallian – shared general architecture with the most ancient of vertebrates embryonic origin – mesencephalon main functions:functions a sort of relay station for sound and visual information serves as a nerve pathway of the cerebral hemispheres controls the eye movement involved in control of body movement Prof. Dr. Nikolai Lazarov 2 Midbrain MidbrainMidbrain –– grossgross anatomyanatomy dorsal part – tectum (quadrigeminal plate): superior colliculi inferior colliculi cerebral aqueduct ventral part – cerebral peduncles:peduncles dorsal – tegmentum (central part) ventral – cerebral crus substantia nigra Prof. Dr. Nikolai Lazarov 3 Midbrain CerebralCerebral cruscrus –– internalinternal structurestructure CerebralCerebral peduncle:peduncle: crus cerebri tegmentum mesencephali substantia nigra two thick semilunar white matter bundles composition – somatotopically arranged motor tracts: corticospinal } pyramidal tracts – medial ⅔ corticobulbar corticopontine fibers: frontopontine tracts – medially temporopontine tracts – laterally -

Gene Expression of Prohormone and Proprotein Convertases in the Rat CNS: a Comparative in Situ Hybridization Analysis
The Journal of Neuroscience, March 1993. 73(3): 1258-1279 Gene Expression of Prohormone and Proprotein Convertases in the Rat CNS: A Comparative in situ Hybridization Analysis Martin K.-H. Schafer,i-a Robert Day,* William E. Cullinan,’ Michel Chri?tien,3 Nabil G. Seidah,* and Stanley J. Watson’ ‘Mental Health Research Institute, University of Michigan, Ann Arbor, Michigan 48109-0720 and J. A. DeSeve Laboratory of *Biochemical and 3Molecular Neuroendocrinology, Clinical Research Institute of Montreal, Montreal, Quebec, Canada H2W lR7 Posttranslational processing of proproteins and prohor- The participation of neuropeptides in the modulation of a va- mones is an essential step in the formation of bioactive riety of CNS functions is well established. Many neuropeptides peptides, which is of particular importance in the nervous are synthesized as inactive precursor proteins, which undergo system. Following a long search for the enzymes responsible an enzymatic cascade of posttranslational processing and mod- for protein precursor cleavage, a family of Kexin/subtilisin- ification events during their intracellular transport before the like convertases known as PCl, PC2, and furin have recently final bioactive products are secreted and act at either pre- or been characterized in mammalian species. Their presence postsynaptic receptors. Initial endoproteolytic cleavage occurs in endocrine and neuroendocrine tissues has been dem- C-terminal to pairs of basic amino acids such as lysine-arginine onstrated. This study examines the mRNA distribution of (Docherty and Steiner, 1982) and is followed by the removal these convertases in the rat CNS and compares their ex- of the basic residues by exopeptidases. Further modifications pression with the previously characterized processing en- can occur in the form of N-terminal acetylation or C-terminal zymes carboxypeptidase E (CPE) and peptidylglycine a-am- amidation, which is essential for the bioactivity of many neu- idating monooxygenase (PAM) using in situ hybridization ropeptides. -
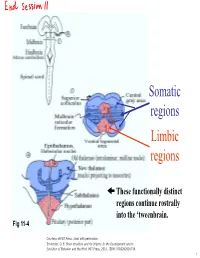
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

Two Fiber Pathways Connecting Amygdala and Prefrontal Cortex in Humans and Monkeys
bioRxiv preprint doi: https://doi.org/10.1101/561811; this version posted March 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Two fiber pathways connecting amygdala and prefrontal cortex in humans and monkeys Davide Folloni1,2*, Jérôme Sallet1,2, Alexandre A. Khrapitchev3, Nicola R. Sibson3, Lennart Verhagen1,2†, Rogier B. Mars2,4† 1Wellcome Integrative Neuroimaging (WIN), Department of Experimental Psychology, University of Oxford, Oxford, United Kingdom 2Wellcome Integrative Neuroimaging (WIN), Centre for Functional MRI of the Brain (FMRIB), Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom 3Cancer Research UK and Medical Research Council Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom 4Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, The Netherlands †Authors contributed equally to the work *To whom correspondence should be addressed: Address: Davide Folloni, Department of Experimental Psychology, University of Oxford, Tinsley Building, Mansfield Road, Oxford, OX1 3SR, UK E-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/561811; this version posted March 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract The interactions between amygdala and prefrontal cortex are pivotal to many neural processes involved in learning, decision-making, emotion, and social regulation. -

Brainstem and Its Associated Cranial Nerves
Brainstem and its Associated Cranial Nerves Anatomical and Physiological Review By Sara Alenezy With appreciation to Noura AlTawil’s significant efforts Midbrain (Mesencephalon) External Anatomy of Midbrain 1. Crus Cerebri (Also known as Basis Pedunculi or Cerebral Peduncles): Large column of descending “Upper Motor Neuron” fibers that is responsible for movement coordination, which are: a. Frontopontine fibers b. Corticospinal fibers Ventral Surface c. Corticobulbar fibers d. Temporo-pontine fibers 2. Interpeduncular Fossa: Separates the Crus Cerebri from the middle. 3. Nerve: 3rd Cranial Nerve (Oculomotor) emerges from the Interpeduncular fossa. 1. Superior Colliculus: Involved with visual reflexes. Dorsal Surface 2. Inferior Colliculus: Involved with auditory reflexes. 3. Nerve: 4th Cranial Nerve (Trochlear) emerges caudally to the Inferior Colliculus after decussating in the superior medullary velum. Internal Anatomy of Midbrain 1. Superior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). a. Tectospinal Pathway: turning the head, neck and eyeballs in response to a visual stimuli.1 Level of b. Spinotectal Pathway: turning the head, neck and eyeballs in response to a cutaneous stimuli.2 Superior 2. Oculomotor Nucleus: Situated in the periaqueductal grey matter. Colliculus 3. Red Nucleus: Red mass3 of grey matter situated centrally in the Tegmentum. Involved in motor control (Rubrospinal Tract). 1. Inferior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). Tectospinal Pathway: turning the head, neck and eyeballs in response to a auditory stimuli. 2. Trochlear Nucleus: Situated in the periaqueductal grey matter. Level of Inferior 3. -

NIH Public Access Author Manuscript Neuromodulation
NIH Public Access Author Manuscript Neuromodulation. Author manuscript; available in PMC 2015 June 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Neuromodulation. 2014 June ; 17(4): 312–319. doi:10.1111/ner.12141. Surgical Neuroanatomy and Programming in Deep Brain Stimulation for Obsessive Compulsive Disorder Takashi Morishita, M.D., Ph.D.1, Sarah M. Fayad, M.D.2, Wayne K. Goodman, M.D.3, Kelly D. Foote, M.D.1, Dennis Chen, B.S.2, David A. Peace, M.S., CMI1, Albert L. Rhoton Jr.1, and Michael S. Okun, M.D.1,2 1Department of Neurosurgery, University of Florida College of Medicine/Shands Hospital, Center for Movement Disorders and Neurorestoration, McKnight Brain Institute, Gainesville, FL Corresponding Author: Takashi Morishita, M.D., Ph.D., Department of Neurosurgery, Mcknight Brain Institute Room L2-100, 1149 South Newell Drive, Gainesville, FL 32611, 352-273-9000, 352-392-8413 FAX, [email protected]. Authorship Statement: Drs. Morishita and Okun deigned and conducted the study, including patient recruitment, data collection and data analysis. Drs. Morishita and Fayad prepared the manuscript draft with important intellectual input from Drs. Okun, Rhoton, Goodman and Foote. Mr. Peace provided his illustration into this manuscript. Mr. Chen contributed to collect the data. All authors approved the final manuscript. Author disclosures 1. Takashi Morishita, M.D., Ph.D. Disclosures: Dr. Morishita has received grant support from Nakatomi foundation, St. Luke’s Life Science Institute of Japan, and Japan Society for Promotion of Science in Japan. 2. Sarah M. -

Functional Neuroanatomy for Posture and Gait Control
REVIEW ARTICLE https://doi.org/10.14802/jmd.16062 / J Mov Disord 2017;10(1):1-17 pISSN 2005-940X / eISSN 2093-4939 Functional ABSTRACT Here we argue functional neuroanatomy for pos- ture-gait control. Multi-sensory information such as Neuroanatomy somatosensory, visual and vestibular sensation act on various areas of the brain so that adaptable pos- ture-gait control can be achieved. Automatic process for Posture and of gait, which is steady-state stepping movements associating with postural reflexes including head- eye coordination accompanied by appropriate align- Gait Control ment of body segments and optimal level of pos- tural muscle tone, is mediated by the descending pathways from the brainstem to the spinal cord. Par- ticularly, reticulospinal pathways arising from the lat- Kaoru Takakusaki eral part of the mesopontine tegmentum and spinal locomotor network contribute to this process. On The Research Center for Brain Function and Medical Engineering, the other hand, walking in unfamiliar circumstance Asahikawa Medical University, Asahikawa, Japan requires cognitive process of postural control, which depends on knowledges of self-body, such as body schema and body motion in space. The cognitive in- formation is produced at the temporoparietal asso- ciation cortex, and is fundamental to sustention of vertical posture and construction of motor programs. The programs in the motor cortical areas run to ex- ecute anticipatory postural adjustment that is opti- mal for achievement of goal-directed movements. The basal ganglia and cerebellum may affect both the automatic and cognitive processes of posture- gait control through reciprocal connections with the brainstem and cerebral cortex, respectively. Conse- quently, impairments in cognitive function by dam- ages in the cerebral cortex, basal ganglia and cere- bellum may disturb posture-gait control, resulting in falling. -

The Three-Dimensional Architecture of the Internal Capsule of the Human Brain Demonstrated by Fiber Dissection Technique
ARS Medica Tomitana - 2014; 3(78): 115 -122 10.2478/arsm-2014-0021 Goga Cristina1,2,3, Brinzaniuc Klara1, Florian I.S.2, Rodriguez Mena R.3 The three-dimensional architecture of the internal capsule of the human brain demonstrated by fiber dissection technique 1. University of Medicine and Pharmacy Tirgu Mures, Tirgu Mures, Romania, Department of Anatomy 2. Cluj County Emergency Hospital, Cluj Napoca, Romania, Department of Neurosurgery 3. Yeditepe University School of Medicine, Istanbul, Turkey, Department of Neurosurgery ABSTRACT Introduction The fiber dissection technique involves peeling away white matter fiber tracts of the brain to display its three-dimensional anatomic arrangement. The intricate three-dimensional configuration and structure of the internal capsule (IC) is not well defined. By using The white matter of the brain consists of the fiber dissection technique, our aim was to expose bundles of myelinated nerve fibers known as and study the IC to achieve a clearer conception of its fascicles or fiber tracts. There are three groups of configuration and relationships with neighboring white nerve fibers: association, connection and projection matter fibers and central nuclei. fibers. Association fibers connect neighboring The lateral and medial aspects of the temporal lobes of and distal cortical region in the same hemisphere. twenty, previously frozen, formalin-fixed human brains Commissural fibers connect homologues regions in were dissected under the operating microscope using the two hemispheres. Projection fibers connect the the fiber dissection technique. The details of the three-dimensional arrangement of the cerebral cortex with subcortical structures such as fibers within the IC were studied and a comprehensive thalamus, basal ganglia, brainstem and spinal cord. -

Compensatory Sprouting and Impulse Rerouting After Unilateral Pyramidal Tract Lesion in Neonatal Rats
The Journal of Neuroscience, September 1, 2000, 20(17):6561–6569 Compensatory Sprouting and Impulse Rerouting after Unilateral Pyramidal Tract Lesion in Neonatal Rats Werner J. Z’Graggen, Karim Fouad, Olivier Raineteau, Gerlinde A. S. Metz, Martin E. Schwab, and Gwendolyn L. Kartje Brain Research Institute, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8057 Zurich, Switzerland After lesions of the developing mammalian CNS, structural plas- tigate possible new functional connections from the motor cortex ticity and functional recovery are much more pronounced than in of the pyramidotomy side to the periphery. In rats lesioned as the mature CNS. We investigated the anatomical reorganization adults, stimulation of the motor cortex ipsilateral to the pyra- of the corticofugal projections rostral to a unilateral lesion of the midotomy never elicited EMG activity. In contrast, in P2 lesioned corticospinal tract at the level of the medullary pyramid (pyra- rats bilateral forelimb EMGs were found. EMG latencies were midotomy) and the contribution of this reorganization and other comparable for the ipsilateral and contralateral responses but descending systems to functional recovery. were significantly longer than in unlesioned animals. Transient Two-day-old (P2) and adult rats underwent a unilateral pyra- inactivation of both red nuclei with the GABA receptor agonist midotomy. Three months later the corticofugal projections to the muscimol led to a complete loss of these bilateral movements. red nucleus and the pons were analyzed; a relatively large num- Movements and EMGs reappeared after wash-out of the drug. ber of corticorubral and corticopontine fibers from the lesioned These results suggest an important role of the red nucleus in the side had crossed the midline and established an additional con- tralateral innervation of the red nucleus and the pons. -

Basal Ganglia and Cerebellum Receive Different Somatosensory Information in Rats (Barrel Field/Pontine Nuclei/Cortical Lamnation/Vibrissae) BARBARA E
Proc. Nati. Acad. Sci. USA Vol. 87, pp. 4388-4392, June 1990 Neurobiology Basal ganglia and cerebellum receive different somatosensory information in rats (barrel field/pontine nuclei/cortical lamnation/vibrissae) BARBARA E. MERCIER*, CHARLES R. LEGGt, AND MITCHELL GLICKSTEIN Department of Anatomy and Developmental Biology, University College London, Gower Street, London WC1, United Kingdom Communicated by Irving T. Diamond, March 14, 1990 ABSTRACT There are two great subcortical circuits that sublamina Vb stain far more densely, suggesting that they are relay sensory information to motor structures in the mamma- capable ofhigher levels oftonic activity than those in Va (12). lian brain. One pathway relays via the pontine nuclei and Previous study of the efferent pathways from the rat cerebellum, and the other relays by way of the basal ganglia. somatosensory cortex (2) has suggested that the cell popu- We studied the cells oforigin ofthese two major pathways from lation projecting to the basal ganglia is centered on lamina Va, the posteromedial barrel subfield ofrats, a distinct region ofthe while the population projecting to the cerebellum is centered somatosensory cortex that contains the sensory representation on Vb. However, the methods available at the time the study of the large whiskers. We itjected tracer substances into the was done were relatively insensitive (13) and a comparison caudate putamen or the pontine nuclei and charted the location with more recent work (14) shows that only a subset of the of retrogradely filled cortical cells. In preliminary studies, we entire population of corticopontine cells was identified. Wise used double-labeling techniques to determine whether the cells and Jones (figure 6a in ref. -

Chapter 3: Internal Anatomy of the Central Nervous System
10353-03_CH03.qxd 8/30/07 1:12 PM Page 82 3 Internal Anatomy of the Central Nervous System LEARNING OBJECTIVES Nuclear structures and fiber tracts related to various functional systems exist side by side at each level of the After studying this chapter, students should be able to: nervous system. Because disease processes in the brain • Identify the shapes of corticospinal fibers at different rarely strike only one anatomic structure or pathway, there neuraxial levels is a tendency for a series of related and unrelated clinical symptoms to emerge after a brain injury. A thorough knowl- • Recognize the ventricular cavity at various neuroaxial edge of the internal brain structures, including their shape, levels size, location, and proximity, makes it easier to understand • Recognize major internal anatomic structures of the their functional significance. In addition, the proximity of spinal cord and describe their functions nuclear structures and fiber tracts explains multiple symp- toms that may develop from a single lesion site. • Recognize important internal anatomic structures of the medulla and explain their functions • Recognize important internal anatomic structures of the ANATOMIC ORIENTATION pons and describe their functions LANDMARKS • Identify important internal anatomic structures of the midbrain and discuss their functions Two distinct anatomic landmarks used for visual orientation to the internal anatomy of the brain are the shapes of the • Recognize important internal anatomic structures of the descending corticospinal fibers and the ventricular cavity forebrain (diencephalon, basal ganglia, and limbic (Fig. 3-1). Both are present throughout the brain, although structures) and describe their functions their shape and size vary as one progresses caudally from the • Follow the continuation of major anatomic structures rostral forebrain (telencephalon) to the caudal brainstem.