Approaches to Macroevolution: 2. Sorting of Variation, Some Overarching Issues, and General Conclusions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessing Symbiont Extinction Risk Using Cophylogenetic Data 2 3 Jorge Doña1 and Kevin P
1 Assessing symbiont extinction risk using cophylogenetic data 2 3 Jorge Doña1 and Kevin P. Johnson1 4 5 1. Illinois Natural History Survey, Prairie Research Institute, University of Illinois at Urbana-Champaign, 6 1816 S. Oak St., Champaign, IL 61820, USA 7 8 *Corresponding authors: Jorge Doña & Kevin Johnson; e-mail: [email protected] & [email protected] 9 10 Abstract: Symbionts have a unique mode of life that has attracted the attention of ecologists and 11 evolutionary biologists for centuries. As a result of this attention, these disciplines have produced 12 a mature body of literature on host-symbiont interactions. In contrast, the discipline of symbiont 13 conservation is still in a foundational stage. Here, we aim to integrate methodologies on symbiont 14 coevolutionary biology with the perspective of conservation. We focus on host-symbiont 15 cophylogenies, because they have been widely used to study symbiont diversification history and 16 contain information on symbiont extinction. However, cophylogenetic information has never been 17 used nor adapted to the perspective of conservation. Here, we propose a new statistic, 18 “cophylogenetic extinction rate” (Ec), based on coevolutionary knowledge, that uses data from 19 event-based cophylogenetic analyses, and which could be informative to assess relative symbiont 20 extinction risks. Finally, we propose potential future research to further develop estimation of 21 symbiont extinction risk from cophylogenetic analyses and continue the integration of this existing 22 knowledge of coevolutionary biology and cophylogenetics into future symbiont conservation 23 studies and practices. 24 25 Keywords: coevolution, coextinction risk, conservation biology, cophylogenies, host-symbiont 26 interactions, parasites. -

Can Darwin's Finches and Their Native Ectoparasites Survive the Control of Th
Insect Conservation and Diversity (2017) 10, 193–199 doi: 10.1111/icad.12219 FORUM & POLICY Coextinction dilemma in the Galapagos Islands: Can Darwin’s finches and their native ectoparasites survive the control of the introduced fly Philornis downsi? 1 2 MARIANA BULGARELLA and RICARDO L. PALMA 1School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand and 2Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand Abstract. 1. The survival of parasites is threatened directly by environmental alter- ation and indirectly by all the threats acting upon their hosts, facing coextinction. 2. The fate of Darwin’s finches and their native ectoparasites in the Galapagos Islands is uncertain because of an introduced avian parasitic fly, Philornis downsi, which could potentially drive them to extinction. 3. We documented all known native ectoparasites of Darwin’s finches. Thir- teen species have been found: nine feather mites, three feather lice and one nest mite. No ticks or fleas have been recorded from them yet. 4. Management options being considered to control P. downsi include the use of the insecticide permethrin in bird nests which would not only kill the invasive fly larvae but the birds’ native ectoparasites too. 5. Parasites should be targeted for conservation in a manner equal to that of their hosts. We recommend steps to consider if permethrin-treated cotton sta- tions are to be deployed in the Galapagos archipelago to manage P. downsi. Key words. Chewing lice, coextinction, Darwin’s finches, dilemma, ectoparasites, feather mites, Galapagos Islands, permethrin, Philornis downsi. Introduction species have closely associated species which are also endangered (Dunn et al., 2009). -

Paradigms for Parasite Conservation
Paradigms for parasite conservation Running Head: Parasite conservation Keywords: parasitology; disease ecology; food webs; economic valuation; ex situ conservation; population viability analysis 1*† 1† 2 2 Eric R. Dougherty , Colin J. Carlson , Veronica M. Bueno , Kevin R. Burgio , Carrie A. Cizauskas3, Christopher F. Clements4, Dana P. Seidel1, Nyeema C. Harris5 1Department of Environmental Science, Policy, and Management, University of California, Berkeley; 130 Mulford Hall, Berkeley, CA, 94720, USA. 2Department of Ecology and Evolutionary Biology, University of Connecticut; 75 N. Eagleville Rd, Storrs, CT, 06269, USA. 3Department of Ecology and Evolutionary Biology, Princeton University; 106A Guyton Hall, Princeton, NJ, 08544, USA. 4Institute of Evolutionary Biology and Environmental Studies, University of Zurich; Winterthurerstrasse 190 CH-8057, Zurich, Switzerland. 5Luc Hoffmann Institute, WWF International 1196, Gland, Switzerland. *email [email protected] † These authors share lead author status This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/cobi.12634. This article is protected by copyright. All rights reserved. Page 2 of 28 Abstract Parasitic species, which depend directly on host species for their survival, represent a major regulatory force in ecosystems and a significant component of Earth’s biodiversity. Yet the negative impacts of parasites observed at the host level have motivated a conservation paradigm of eradication, moving us further from attainment of taxonomically unbiased conservation goals. Despite a growing body of literature highlighting the importance of parasite-inclusive conservation, most parasite species remain understudied, underfunded, and underappreciated. -

Forecasting Extinctions: Uncertainties and Limitations
Diversity 2009, 1, 133-150; doi:10.3390/d1020133 OPEN ACCESS diversity ISSN 2071-1050 www.mdpi.com/journal/diversity Review Forecasting Extinctions: Uncertainties and Limitations Richard J. Ladle 1,2 1 School of Geography and the Environment, Oxford University, South Parks Road, Oxford, UK; E-Mail: [email protected]; Tel.: +55-31-3899 1902; Fax: +55-31-3899-2735 2 Department of Agricultural and Environmental Engineering, Federal University of Viçosa, Viçosa, Brazil Received: 13 October 2009 / Accepted: 14 November 2009 / Published: 26 November 2009 Abstract: Extinction forecasting is one of the most important and challenging areas of conservation biology. Overestimates of extinction rates or the extinction risk of a particular species instigate accusations of hype and overblown conservation rhetoric. Conversely, underestimates may result in limited resources being allocated to other species/habitats perceived as being at greater risk. In this paper I review extinction models and identify the key sources of uncertainty for each. All reviewed methods which claim to estimate extinction probabilities have severe limitations, independent of if they are based on ecological theory or on rather subjective expert judgments. Keywords: extinction; uncertainty; forecasting; local extinction; viability 1. Introduction ―Prediction is very difficult, especially about the future‖ Nils Bohr, Nobel Prize winning physicist Preventing the extinction of species is probably the most emblematic objective of the global conservation movement. To fulfill this aim effectively requires that decision makers and environmental managers are provided with accurate information on: (1) the identities of specific species/populations with a high probability of going extinct without further interventions; (2) the predicted rates of extinction among a range of taxa in different geographic areas and biomes under various ecological scenarios. -

Genome Size and the Extinction of Small Populations
bioRxiv preprint doi: https://doi.org/10.1101/173690; this version posted August 8, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Genome size and the extinction of small populations Thomas LaBar1;2;3, Christoph Adami1;2;3;4 1 Department of Microbiology & Molecular Genetics 2 BEACON Center for the Study of Evolution in Action 3 Program in Ecology, Evolutionary Biology, and Behavior 4 Department of Physics and Astronomy Michigan State University, East Lansing, MI 48824 Abstract Species extinction is ubiquitous throughout the history of life. Understanding the factors that cause some species to go extinct while others survive will help us manage Earth's present-day biodiversity. Most studies of extinction focus on inferring causal factors from past extinction events, but these studies are constrained by our inability to observe extinction events as they occur. Here, we use digital experimental evolution to avoid these constraints and study \extinction in action". Previous digital evolution experiments have shown that strong genetic drift in small populations led to larger genomes, greater phenotypic complexity, and high extinction rates. Here we show that this elevated extinction rate is a consequence of genome expansions and a con- current increase in the genomic mutation rate. High genomic mutation rates increase the lethal mutation rate, leading to an increase the likelihood of a mutational melt- down. Genome expansions contribute to this lethal mutational load because genome expansions increase the likelihood of lethal mutations. -

Extinctions, Modern Examples Of
EXTINCTIONS, MODERN EXAMPLES OF Ga´bor. L. Lo¨ vei University of Aarhus I. Species Life Spans can trigger a chain of extinctions, the ‘‘B.’’ For ex- II. Examples, Postglacial ample, when the passenger pigeon died out, at least III. Examples, First-Contact Extinctions two feather lice parasites followed it into extinc- IV. Examples, Historical Extinctions (1600–) tion. ‘‘Coextinction’’ occurs when more than one V. Examples, Extinction Cascades species dies out at the same time (this by definition VI. Problems in our Understanding of must happen when the host of an obligate parasite Extinctions dies out). VII. Theoretical Aspects of Extinction first-contact extinctions A wave of extinction of spe- VIII. The Present: A Full-Fledged Mass cies native to a continent or island, following the Extinction first arrival of humans to that area. living dead A term coined by the American tropical biologist, Daniel Janzen, denoting the last living individuals of a species destined to extinction. By GLOSSARY definition, extinction happens when the last individual of a species dies. In reality, however, ex- extinction Disappearance of the last living individual tinction of a species can be certain even earlier. of a species. Extinction can be ‘‘local B,’’ if it con- Most species need both males and females to re- cerns a definite population or location; we speak of produce. If there are no fertile individuals of one ‘‘B in the wild’’ when the only individuals alive of a sex, the species is doomed to extinction even if species are in captivity; and of ‘‘global B’’ when no several individuals are still alive. -
Geologic Time Scale
Geologic time scale – Chronology of Earth’s history – 4.7 billion history of the earth from its origin to the present – Transitions in the fossil record, found in characteristic layers of sedimentary rock – Formulated by assessing the age of rocks and rock sediments. – Correlates with evolutionary events Geologic time scale Based on geologic events the ancient period from earth’s history is formulated into eons-eras-periods-epochs. Each division in the geological calendar is clearly identified and described. Incidents pertaining to earth surface, plant and animal life are neatly recorded. The influence of geological and climatic changes on the life and the evolution of the living organism had been well analyzed. Earth is 4.7 billion (4,700 million) years old. Geologic time (4.7 billion/4,700 million) Divides geologic history into Divisions: four-level hierarchy of time intervals units • EONS Originally created using relative First and largest division of geologic time dates and more recently, Greatest expanse of time radioactive dating. Four eons The influence of geological and • Phanerozoic ("visible life") –most recent eon climatic changes on the life and • Proterozoic the evolution of the living • Archean organism • Hadean – the oldest eon • ERAS Second division of geologic time • PERIODS Third division of the geologic time. Named for either location or characteristics of the defining rock formations • EPOCHS Fourth division of geologic time Represents the subdivisions of a period . The geologic scale time The geologic PRECAMBRIAN SUPER EON 1. HADEAN EON (PRE-ARCHEAN EON) 4.6 to 3.8 billion years ago ~4.6 BYA -- Formation of Earth and Moon (as indicated by dating of meteorites and rocks from the Moon) ~4 BYA -- Likely origin of life -- indirect photosynthetic evidence of primordial life -- evidence of materials created by organic decay This is the "hidden" portion of geologic time as there is little evidence of this time remaining in Earth's rocks. -

The Indirect Paths to Cascading Effects of Extinctions in Mutualistic Networks
UC Merced UC Merced Previously Published Works Title The indirect paths to cascading effects of extinctions in mutualistic networks. Permalink https://escholarship.org/uc/item/4m9305hh Journal Ecology, 101(7) ISSN 0012-9658 Authors Pires, Mathias M O'Donnell, James L Burkle, Laura A et al. Publication Date 2020-07-01 DOI 10.1002/ecy.3080 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Reports Ecology, 101(7), 2020, e03080 © 2020 by the Ecological Society of America The indirect paths to cascading effects of extinctions in mutualistic networks 1,11 2 3 4 MATHIAS M. PIRES , JAMES L. O'DONNELL, LAURA A. BURKLE , CECILIA DIAZ-CASTELAZO , 5,6 7 6 8 DAVID H. HEMBRY , JUSTIN D. YEAKEL , ERICA A. NEWMAN , LUCAS P. M EDEIROS , 9 10 MARCUS A. M. DE AGUIAR , AND PAULO R. GUIMARAES~ JR. 1Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas, Campinas,13.083-862, Sao~ Paulo, Brazil 2School of Marine and Environmental Affairs, University of Washington, Seattle, WA 98105, Washington, USA 3Department of Ecology, Montana State University, Bozeman, MT 59717, Montana, USA 4Red de Interacciones Multitroficas, Instituto de Ecologıa, A.C., Xalapa, VER 11 351, Veracruz, Mexico 5Department of Entomology, Cornell University, Ithaca, NY 14853, New York, USA 6Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, Arizona, USA 7School of Natural Sciences, University of California, Merced, CA 95343, California, USA 8Department of Civil and Environmental Engineering, MIT, Cambridge, MA 02142, Massachusetts, USA 9Instituto de Fısica “Gleb Wataghin”, Universidade Estadual de Campinas, Campinas, 13083-859, Sao~ Paulo, Brazil 10Departamento de Ecologia, Instituto de Bioci^encias, Universidade de Sao~ Paulo, Sao~ Paulo 05508-090, Brazil Citation: Pires, M. -

Extinctions and Biodiversity in the Fossil Record
Extinctions and Biodiversity in the Fossil Record Ricard V Sole´ and Mark Newman Volume 2, The Earth system: biological and ecological dimensions of global environmental change, pp 297–301 Edited by Professor Harold A Mooney and Dr Josep G Canadell in Encyclopedia of Global Environmental Change (ISBN 0-471-97796-9) Editor-in-Chief Ted Munn John Wiley & Sons, Ltd, Chichester, 2002 wiped out the dinosaurs along with about 70% of all other Extinctions and Biodiversity species then alive. The interval from the KT event until the in the Fossil Record present, known as the Cenozoic eon, saw the radiation of the mammals to fill many of the dominant land-dwelling niches and, eventually, the evolution of mankind. Ricard V SoleandMarkNewman ´ This information comes from geological studies and Santa Fe Institute, Santa Fe, NM, USA from the substantial fossil record of extinct lifeforms. The currently known fossil record includes about one-quarter of a million species, mostly dating from the time interval Life has existed on Earth for more than three billion years. between the Cambrian explosion and the present. There Until the Cambrian explosion about 540 million years ago, are numerous biases in the fossil record, which make however, life was restricted mostly to single-celled microor- accurate quantitative investigations difficult, including the ganisms that were, for the most part, poorly preserved in following. the fossil record. From the Cambrian explosion onwards, by contrast, we have a substantial fossil record of life’s devel- 1. Older fossils are harder to find because they are opment, which shows a number of clear patterns, including typically buried in deeper rocks than more recent ones. -
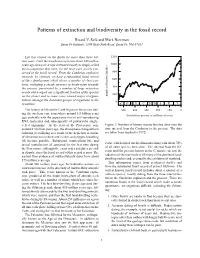
Patterns of Extinction and Biodiversity in the Fossil Record
Patterns of extinction and biodiversity in the fossil record Ricard V. Sol´e and Mark Newman Santa Fe Institute, 1399 Hyde Park Road, Santa Fe, NM 87501 Life has existed on the Earth for more than three bil- 1200 lion years. Until the Cambrian explosion about 540 million years ago however, it was restricted mostly to single-celled 1000 micro-organisms that were, for the most part, poorly pre- served in the fossil record. From the Cambrian explosion 800 onwards, by contrast, we have a substantial fossil record of life’s development which shows a number of clear pat- 600 terns, including a steady increase in biodiversity towards 400 the present, punctuated by a number of large extinction events which wiped out a significant fraction of the species 200 on the planet and in some cases caused major reorgani- number of known families zations amongst the dominant groups of organisms in the 0 ecosphere. −C OSD C PTrJ K T The history of life on the Earth begins in the oceans dur- 500 400 300 200 100 0 ing the Archean eon, somewhere around 3.8 billion years time before present in millions of years ago, probably with the appearance first of self-reproducing RNA molecules and subsequently of prokaryotic single- celled organisms. At the start of the Proterozoic eon, Figure 1: Number of known marine families alive over the around 2.5 billion years ago, the atmosphere changed from time interval from the Cambrian to the present. The data reducing to oxidizing as a result of the depletion of stocks are taken from Sepkoski (1992). -

Are Generalist Parasites Being Lost from Their Hosts?
Journal of Animal Ecology 2016 doi: 10.1111/1365-2656.12470 FORUM Response to Strona & Fattorini: are generalist parasites being lost from their hosts? Maxwell J. Farrell1*, Patrick R. Stephens2 and T. Jonathan Davies1,3 1Biology Department, McGill University, 1205 Docteur Penfield, Montreal, QC H3A 1B1, Canada; 2Odum School of Ecology, University of Georgia, Athens, GA 30602, USA; and 3African Centre for DNA Barcoding, University of Johannesburg, PO Box 524, Auckland Park, 2006 Johannesburg, South Africa Key-words: asymmetry of interactions, coextinction, conservation, host range, host speci- ficity, infectious disease, macroecology, parasite richness 3 The inclusion of both host life-history traits and mea- Introduction sures of current extinction risk as predictors in our Strona & Fattorini (2015) raise concerns about the con- analyses was not properly justified and could lead to clusions of a recent analysis by Farrell et al. (2015) on contrasting results if we were attempting to explain patterns of host–parasite associations among free-living the evolution of parasite specialization. carnivores and terrestrial ungulates. Contrary to expecta- We believe these criticisms arise from a few simple tions from coextinction theory, our study found ungulate misunderstandings; we address each point in detail below. species threatened with extinction are associated with a higher proportion of single-host parasites compared to non-threatened species. The increased proportion of sin- gle-host parasites in threatened ungulates appears to Non-random distributions of specialist and result from decreased richness of multi-host parasites as generalist parasites we do not observe significant differences in the richness of In various species interaction networks, non-random dis- single-host parasites between threatened and non-threa- tributions of specialist and generalist affiliates have been tened ungulates. -
Coextinction and Persistence of Dependent Species in a Changing World
ES43CH09-Colwell ARI 26 September 2012 11:4 Coextinction and Persistence of Dependent Species in a Changing World Robert K. Colwell,1,2 Robert R. Dunn,3 and Nyeema C. Harris4 1Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, Connecticut 06269; email: [email protected] 2University of Colorado Museum of Natural History, Boulder, Colorado 80309 3Department of Biology and 4Department of Forestry and Environmental Resources, North Carolina State University, Raleigh, North Carolina 27607; email: [email protected] Annu. Rev. Ecol. Evol. Syst. 2012. 43:183–203 Keywords First published online as a Review in Advance on affiliate species, commensalism, extinction cascade, extinction vortex, food August 28, 2012 web, host switching, interaction network, mutualism, parasitism, The Annual Review of Ecology, Evolution, and pollination, secondary extinction Systematics is online at ecolsys.annualreviews.org This article’s doi: Abstract 10.1146/annurev-ecolsys-110411-160304 The extinction of a single species is rarely an isolated event. Instead, depen- Copyright c 2012 by Annual Reviews. dent parasites, commensals, and mutualist partners (affiliates) face the risk by North Carolina State University on 11/12/14. For personal use only. All rights reserved of coextinction as their hosts or partners decline and fail. Species interac- 1543-592X/12/1201-0183$20.00 tions in ecological networks can transmit the effects of primary extinctions Annu. Rev. Ecol. Evol. Syst. 2012.43:183-203. Downloaded from www.annualreviews.org within and between trophic levels, causing secondary extinctions and extinc- tion cascades. Documenting coextinctions is complicated by ignorance of host specificity, limitations of historical collections, incomplete systematics of affiliate taxa, and lack of experimental studies.