Hidden Hassium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
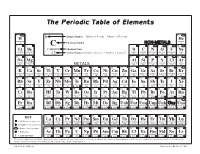
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

The Oganesson Odyssey Kit Chapman Explores the Voyage to the Discovery of Element 118, the Pioneer Chemist It Is Named After, and False Claims Made Along the Way
in your element The oganesson odyssey Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way. aving an element named after you Ninov had been dismissed from Berkeley for is incredibly rare. In fact, to be scientific misconduct in May5, and had filed Hhonoured in this manner during a grievance procedure6. your lifetime has only happened to Today, the discovery of the last element two scientists — Glenn Seaborg and of the periodic table as we know it is Yuri Oganessian. Yet, on meeting Oganessian undisputed, but its structure and properties it seems fitting. A colleague of his once remain a mystery. No chemistry has been told me that when he first arrived in the performed on this radioactive giant: 294Og halls of Oganessian’s programme at the has a half-life of less than a millisecond Joint Institute for Nuclear Research (JINR) before it succumbs to α -decay. in Dubna, Russia, it was unlike anything Theoretical models however suggest it he’d ever experienced. Forget the 2,000 ton may not conform to the periodic trends. As magnets, the beam lines and the brand new a noble gas, you would expect oganesson cyclotron being installed designed to hunt to have closed valence shells, ending with for elements 119 and 120, the difference a filled 7s27p6 configuration. But in 2017, a was Oganessian: “When you come to work US–New Zealand collaboration predicted for Yuri, it’s not like a lab,” he explained. that isn’t the case7. -

Atomic Properties of the Elements
P E R I O D I C T A B L E Group 1 18 IA Atomic Properties of the Elements VIIIA 2 1 S FREQUENTLY USED FUNDAMENTAL PHYSICAL CONSTANTS§ S 1 1/2 Physical Measurement Laboratory www.nist.gov/pml 2 0 1 second = 9 192 631 770 periods of radiation corresponding to the H transition between the two hyperfine levels of the ground state of 133Cs Standard Reference Data www.nist.gov/srd He 1 Hydrogen −1 § For the most accurate Helium speed of light in vacuum c 299 792 458 m s (exact) values of these and 1.008* −34 4.002602 2 1s 2 Planck constant h 6.626 070 x 10 J s ( ħ /2 ) other constants, visit 13 14 15 16 17 1s −19 13.5984 IIA elementary charge e 1.602 177 x 10 C physics.nist.gov/constants IIIA IVA VA VIA VIIA 24.5874 −31 2 1 electron mass me 9.109 384 x 10 kg 2 3 4 3 2 1 3 S1/2 4 S0 2 5 P°1/2 6 P0 7 S3/2° 8 P2 9 P3/2° 10 S0 mec 0.510 999 MeV −27 Solids Li Be proton mass mp 1.672 622 x 10 kg B C N O F Ne 2 Lithium Beryllium fine-structure constant 1/137.035 999 Liquids Boron Carbon Nitrogen Oxygen Fluorine Neon 6.94* 9.0121831 −1 10.81* 12.011* 14.007* 15.999* 18.99840316* 20.1797 Rydberg constant R 10 973 731.569 m 2 2 2 Gases 2 2 2 2 2 2 2 3 2 2 4 2 2 5 2 2 6 15 1s 2s 2p 1s 2s 1s 2s 1s 2s 1s 2s 1s 2s 1s 2s R c 3.289 841 960 x 10 Hz 2p 2p 1s 2s 2p 2p 2p 5.3917 9.3227 Artificially 8.2980 11.2603 14.5341 13.6181 17.4228 21.5645 R hc 13.605 693 eV 2 1 -19 Prepared 2 3 4 3 2 1 11 S1/2 12 S0 electron volt eV 1.602 176 6 x 10 J 13 P1/2° 14 P0 15 S3/2° 16 P2 17 P3/2° 18 S0 −23 −1 Boltzmann constant k 1.380 65 x 10 J K −1 −1 Na Mg molar gas constant -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability. -

The Quest to Explore the Heaviest Elements Raises Questions About How Far Researchers Can Extend Mendeleev’S Creation
ON THE EDGE OF THE PERIODIC TABLE The quest to explore the heaviest elements raises questions about how far researchers can extend Mendeleev’s creation. BY PHILIP BALL 552 | NATURE | VOL 565 | 31 JANUARY 2019 ©2019 Spri nger Nature Li mited. All ri ghts reserved. ©2019 Spri nger Nature Li mited. All ri ghts reserved. FEATURE NEWS f you wanted to create the world’s next undiscovered element, num- Berkeley or at the Joint Institute for Nuclear Research (JINR) in Dubna, ber 119 in the periodic table, here’s a possible recipe. Take a few Russia — the group that Oganessian leads — it took place in an atmos- milligrams of berkelium, a rare radioactive metal that can be made phere of cold-war competition. In the 1980s, Germany joined the race; I only in specialized nuclear reactors. Bombard the sample with a beam an institute in Darmstadt now named the Helmholtz Center for Heavy of titanium ions, accelerated to around one-tenth the speed of light. Ion Research (GSI) made all the elements between 107 and 112. Keep this up for about a year, and be patient. Very patient. For every The competitive edge of earlier years has waned, says Christoph 10 quintillion (1018) titanium ions that slam into the berkelium target Düllmann, who heads the GSI’s superheavy-elements department: — roughly a year’s worth of beam time — the experiment will probably now, researchers frequently talk to each other and carry out some produce only one atom of element 119. experiments collaboratively. The credit for creating later elements On that rare occasion, a titanium and a berkelium nucleus will collide up to 118 has gone variously, and sometimes jointly, to teams from and merge, the speed of their impact overcoming their electrical repul- sion to create something never before seen on Earth, maybe even in the Universe. -

Periodic Table Expands with Elements Named After Japan, Moscow, Tennessee 1 December 2016
Periodic table expands with elements named after Japan, Moscow, Tennessee 1 December 2016 including nihonium—official symbol Nh—are synthesised in laboratories. All the discovered elements after 104 are synthetic ones produced through laboratory experiments. Tradition dictates that newly discovered elements be named after a place, geographical region, or scientist, according to the International Union of Pure and Applied Chemistry (IUPAC), which made the announcement on the names Wednesday. A joint team of Russian and US scientists named element 115 moscovium—symbol Mc—after the Russian capital, where much of the relevant research was conducted. For similar reasons, they also named element 117 tennessine—symbol Ts—after the US state of Credit: CC0 Public Domain Tennessee. The periodic table got larger Wednesday after four The third one they discovered, element 118, was new elements were officially named and added to named oganesson—symbol Og—in homage to the chart, including 'nihonium'—the first ever to be Russian nuclear physicist Yuri Oganessian, in discovered by Japanese scientists. recognition of his "pioneering contributions" in elements research. The new name for element 113, a highly radioactive element with an extremely short half- North Carolina-based IUPAC said that the names life, comes from Japan's name in were officially accepted after a 5-month public Japanese—'nihon', literally 'the land of the rising review period. sun'. "The names of the new elements reflect the "The element, named for the first time by Japanese realities of our present time," IUPAC president and in Asia, will occupy a place in the periodic Natalia Tarasova said on its website, citing the table—an intellectual asset of mankind," Kosuke "universality of science" as well as "the pivotal role" Morita, who led the team that created the element, of Oganessian. -

Chemical Investigations of Element 108, Hassium (Hs)
Lawrence Berkeley National Laboratory Lawrence Berkeley National Laboratory Title Chemical investigations of Element 108, Hassium (Hs) Permalink https://escholarship.org/uc/item/4p69z3hd Author Dullmann, Christoph E. Publication Date 2003-07-03 eScholarship.org Powered by the California Digital Library University of California This abstract was prepared for an invited oral presentation at the “2nd International Conference on the Chemistry and Physics of the Transactinide Elements”, TAN03, in Napa, CA, November 16- 20, 2003. prepared: July 3, 2003 LBNL-52278 Ext. Abs. Chemical investigations of element 108, hassium (Hs) Ch.E. Düllmann1,2 1Lawrence Berkeley National Laboratory, Berkeley, USA 2University of California, Berkeley, USA [email protected] INTRODUCTION The basic aim of chemistry experiments of transactinide elements (TAN) is to establish their place in the periodic table of the elements, i.e. to determine if their chemical behavior is similar to the one of supposed homologs. In this contribution I will try to give an overview of all chemical experiments on element 108, hassium (Hs) that have been reported to date. Based on the systematics of the periodic table, Hs is expected to be a member of group 8 and therefore homologous to osmium (Os) and ruthenium (Ru). As a member of the transactinide series, its experimental investigation is complicated by low production cross-sections and short half-lives. It has therefore been successfully investigated only recently. Already in the seventies of the last century, several authors mentioned the tetroxides of the two heavier group 8 elements, Ru and Os, to be very outstanding compounds with respect to their unusually high volatility. -

Upper Limit of the Periodic Table and the Future Superheavy Elements
CLASSROOM Rajarshi Ghosh Upper Limit of the Periodic Table and the Future Department of Chemistry The University of Burdwan ∗ Superheavy Elements Burdwan 713 104, India. Email: [email protected] Controversy surrounds the isolation and stability of the fu- ture transactinoid elements (after oganesson) in the periodic table. A single conclusion has not yet been drawn for the highest possible atomic number, though there are several the- oretical as well as experimental results regarding this. In this article, the scientific backgrounds of those upcoming super- heavy elements (SHE) and their proposed electronic charac- ters are briefly described. Introduction Totally 118 elements, starting from hydrogen (atomic number 1) to oganesson (atomic number 118) are accommodated in the mod- ern form of the periodic table comprising seven periods and eigh- teen groups. Total 92 natural elements (if technetium is consid- ered as natural) are there in the periodic table (up to uranium hav- ing atomic number 92). In the actinoid series, only four elements— Keywords actinium, thorium, protactinium and uranium—are natural. The Superheavy elements, actinoid rest of the eleven elements—from neptunium (atomic number 93) series, transactinoid elements, periodic table. to lawrencium (atomic number 103)—are synthetic. Elements after actinoids (i.e., from rutherfordium) are called transactinoid elements. These are also called superheavy elements (SHE) as they have very high atomic numbers. Prof. G T Seaborg had Elements after actinoids a very distinct contribution in the field of transuranium element (i.e., from synthesis. For this, Prof. Seaborg was awarded the Nobel Prize in rutherfordium) are called transactinoid elements. 1951. -

Critical Mineral Resources of the United States— Economic and Environmental Geology and Prospects for Future Supply
Critical Mineral Resources of the United States— Economic and Environmental Geology and Prospects for Future Supply Professional Paper 1802 U.S. Department of the Interior U.S. Geological Survey Periodic Table of Elements 1A 8A 1 2 hydrogen helium 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 lithium beryllium boron carbon nitrogen oxygen fluorine neon 6.94 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 13 14 15 16 17 18 sodium magnesium aluminum silicon phosphorus sulfur chlorine argon 22.99 24.31 3B 4B 5B 6B 7B 8B 11B 12B 26.98 28.09 30.97 32.06 35.45 39.95 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 potassium calcium scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc gallium germanium arsenic selenium bromine krypton 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 72.64 74.92 78.96 79.90 83.79 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 rubidium strontium yttrium zirconium niobium molybdenum technetium ruthenium rhodium palladium silver cadmium indium tin antimony tellurium iodine xenon 85.47 87.62 88.91 91.22 92.91 95.96 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 cesium barium hafnium tantalum tungsten rhenium osmium iridium platinum gold mercury thallium lead bismuth polonium astatine radon 132.9 137.3 178.5 180.9 183.9 186.2 190.2 192.2 195.1 197.0 200.5 204.4 207.2 209.0 (209) (210)(222) 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 francium radium rutherfordium