(12) Patent Application Publication (10) Pub. No.: US 2016/0290223 A1 Mills (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Megalomania's Controversial Chem Lab
Megalomania's Controversial Chem Lab Navigation Welcome to the Controversial Chem Lab. Here at the Chem Lab you » Home can find information on a large number of chemicals that have a » Explosives certain stigma attached to them. Chemicals such as explosives, drugs, and pesticides are vitally important for the survival of our civilization. » Chemical Unfortunately, the scientific elite jealously hoards the knowledge on Weapons using and preparing these chemicals. Adding to the confusion is the » Pharmaceuticals scientific ignorant who fear chemistry and think these chemicals are » Pesticides dangerous. As my chemistry professor used to say about what they think, “chemistry equals bad.” » Precursors The Controversial Chem Lab was created to be a free reference on » Lab Skills how to synthesize chemicals. It is also a virtual laboratory skills » Lab Equipment manual, complete with descriptions on how to conduct laboratories, » Safety and a visual database on many different kinds of laboratory apparatus. While the Chem Lab is written for the non-chemist audience, it does » Rogue Science require a basic understanding of laboratory skills. Of course, all of the » Links information needed to acquire a basic understanding of lab skills is » What’s New included within the site. The Chem Lab even goes the extra mile in providing information on » Contact Me how to synthesize many of the chemicals used in making explosives, » Disclaimer etc. It also provides information on where to acquire certain chemicals » Search this site and apparatus. While all of this information is perfectly legal, it may be against the law in certain areas to prepare some of these chemicals without the proper license. -

WO 201 1/056841 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number ι (43) International Publication Date ¾ ί t 12 May 2011 (12.05.2011) WO 201 1/056841 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07C 213/02 (2006.01) C07D 271/12 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US2010/055248 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 3 November 2010 (03.1 1.2010) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/257,721 3 November 2009 (03.1 1.2009) U S (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): PACIF¬ GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, IC SCIENTIFIC ENERGETIC MATERIALS COM¬ ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, PANY [US/US]; 7073 W . -

Metallic Salts, Chemical Dyes, Ingredients That Have Quasi-Spiritual Claims, and Para-Phenylenediamine to Create a Range of Colors
Compound henna: Part 1 Henna, lawsonia inermis, has ONE translucent dye color, rusty red-orange, based on the lawsone molecule precursors naturally produced in the henna leaf. The commercially available henna hair dyes that come in “colors,” such as black, brunette, chestnut, blonde, and so on, are compound hennas. These products have additives to change the color of henna. The additives may be other plants, toxic metallic salts, chemical dyes, ingredients that have quasi-spiritual claims, and para-phenylenediamine to create a range of colors. These hair dyes often contain little henna, or possibly even no henna whatsoever. Sometimes these compound henna dye additives are harmless, others are dangerous. If the additives and adulterants are not declared, they can cause all sorts of health problems as well as destructive cross-reactions if they come in contact with the chemicals used in oxidative dyes. The added ingredients in compound hennas are often not listed or the declarations may be fallacious if the countries of origin do not require declarations for cosmetics, or if the manufacturer chooses to obfuscate for profit. If exported to the west; there is no requirement that the additives be discovered and declared. The pre-packaged henna compounds are often termed “natural herbal henna.” This is misleading as these are not “natural” products; they are full of synthetic chemicals. Metallic salts alter and fix color in lieu of higher quality henna. The compounds of henna and metallic salts can react disastrously with synthetic hair dye, seriously damaging hair. The most frequently used material is lead acetate, though silver nitrate, copper, nickel, cobalt, bismuth and iron salts have also been used. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0072836A1 Mills (43) Pub
US 20140072836A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0072836A1 Mills (43) Pub. Date: Mar. 13, 2014 (54) H2O-BASED ELECTROCHEMICAL (52) U.S. Cl. HYDROGEN-CATALYST POWER SYSTEM CPC ....................................... H0IM 8/06 (2013.01) USPC ............................................... 429/8; 429/422 (75) Inventor: Randell Lee Mills, Cranbury, NJ (US) (57) ABSTRACT (73) Assignee: BLACKLIGHT POWER, INC., An electrochemical power system is provided that generates Cranbury, NJ (US) an electromotive force (EMF) from the catalytic reaction of Appl. No.: 14/005,851 hydrogen to lower energy (hydrino) states providing direct (21) conversion of the energy released from the hydrino reaction (22) PCT Filed: Mar. 30, 2012 into electricity, the system comprising at least two compo nents chosen from: H2O catalyst or a source of H2O catalyst; (86) PCT NO.: PCT/US12A31639 atomic hydrogen or a source of atomic hydrogen; reactants to form the H2O catalyst or source of H2O catalyst and atomic S371 (c)(1), hydrogen or source of atomic hydrogen; and one or more (2), (4) Date: Nov. 21, 2013 reactants to initiate the catalysis of atomic hydrogen. The electrochemical power system for forming hydrinos and elec Related U.S. Application Data tricity can further comprise a cathode compartment compris (60) Provisional application No. 61/472,076, filed on Apr. ing a cathode, an anode compartment comprising an anode, 5, 2011, provisional application No. 61/482.932, filed optionally a saltbridge, reactants that constitute hydrino reac on May 5, 2011, provisional application No. 61/485, tants during cell operation with separate electron flow and ion 769, filed on May 13, 2011, provisional application mass transport, and a source of hydrogen. -

Glossary of Combustion
Glossary of Combustion Maximilian Lackner Download free books at Maximilian Lackner Glossary of Combustion 2 Download free eBooks at bookboon.com Glossary of Combustion 2nd edition © 2014 Maximilian Lackner & bookboon.com ISBN 978-87-403-0637-8 3 Download free eBooks at bookboon.com Glossary of Combustion Contents Contents Preface 5 1 Glossary of Combustion 6 2 Books 263 3 Papers 273 4 Standards, Patents and Weblinks 280 5 Further books by the author 288 4 Click on the ad to read more Download free eBooks at bookboon.com Glossary of Combustion Preface Preface Dear Reader, In this glossary, more than 2,500 terms from combustion and related fields are described. Many of them come with a reference so that the interested reader can go deeper. The terms are translated into the Hungarian, German, and Slovak language, as Central and Eastern Europe is a growing community very much engaged in combustion activities. Relevant expressions were selected, ranging from laboratory applications to large-scale boilers, from experimental research such as spectroscopy to computer simulations, and from fundamentals to novel developments such as CO2 sequestration and polygeneration. Thereby, students, scientists, technicians and engineers will benefit from this book, which can serve as a handy aid both for academic researchers and practitioners in the field. This book is the 2nd edition. The first edition was written by the author together with Harald Holzapfel, Tomás Suchý, Pál Szentannai and Franz Winter in 2009. The publisher was ProcessEng Engineering GmbH (ISBN: 978-3902655011). Their contribution is acknowledged. Recommended textbook on combustion: Maximilian Lackner, Árpád B. -

Practical Lab Manual of Pharmaceutical Organic Chemistry - I
Practical Lab Manual of Pharmaceutical Organic Chemistry - I As Per PCI Syllabus B. Pharm 2nd Semester Dr. Shivendra Kumar Dwivedi M. Pharm (Pharmaceutical Chemsitry), Ph.D. Assoc. Professor University of Pharmacy, Oriental University, Indore (MP) IP Innovative Publication Pvt. Ltd. Dedicated Affectionately to my Father Mr. Ramkhelawan Dwivedi, he is also a great teacher in a sky and my Mother Mrs. Phool Kumari Dwivedi. Shivendra Kumar Dwivedi About the Author Dr. Shivendra Kumar Dwivedi, M. Pharm (Pharmaceutical Chemistry), Ph.D., presently working as an Assoc. Professor in University Institute of Pharmacy, Oriental University, Indore (M.P). He has 10 years of experience in academics and research. He is also the author of some of the other books for UG and PG in Pharmaceutical Chemistry and Practical manual. He has published more than 30 research papers in a different versatile International and National journals. Acknowledgement I am indebted to all my family members particularly to my wife Mrs. Namrata Dwivedi, my daughter Shravi Dwivedi who have always remained my source of inspiration and encouragement. I am grateful to Dr. Neetesh Jain, Principal, UIP, Oriental University, Indore (M.P.) for every step to encourage to publish the book. I am also grateful to Dr. Mahavir Chached, Principal, OCPR, Oriental University, Indore (M.P.) and Dr. Rakesh Patel, Principal & Professor, School of Pharmacy, APJ Abdul Kalam University, Indore (M.P.) for giving guidance to publish the book. Note for the Students If you are a student, you will probably appreciate our effort to present you the book “Practical Lab manual of Pharmaceutical Organic Chemistry - I, which covers all practicals in the 2nd semester in organic chemistry - I. -

FSTC 381-5042, Handbook of Foreign Explosives
U. S. ARMY MATERIEL COMMAND FSIC 381-5042 HANDBOOK OF FOREIGN EXPLOSIVES U.S. Army Foreign Science and Technology Center Munitions Building, Washington, D.C. 20315 FSTC 381-5CA-2 is published for the information and guidance of all concerned. Comments, and requests for additional copies, should he sent to the Commanding Officer, at the above address. A Category I Intelligence Document. This document was compiled and pro ducted by the U.S. Army Foreign Science and Technology Center and is intended for rise within the U.S. Army Materiel Command. This document has not been approved by the Office of the Assistant Chief of Staff for Intelligence and therefore does not necessarily represent agreed Depart ment of the Army intelligence. DESCRIPTORS Explosives: manufacturing, testing, use, characteristics, loading, com parison, strength power, brisance, composition; pyrotechnics; bursting charge; propelling charge; high-explosive ammunition. Project Eld 596 FSTC 381-5042 HANDBOOK OF FOREIGN EXPLOSIVES October 1965 (Based on information available as of June 1964) Prepared by Picatinny Arsenal Dover, New Jersey 07801 for ' U.S. Army Foreign Science and Technology Center Munitions Building Washington, D. C . 20315 ABSTRACT (u) This Handbook of Foreign Explosives contains technical information and reference data on the chemical and physical character istics, known variations in nomenclature, and the application of explosive compounds to the various types of ammunition in use by selected countries. TABLE OF CONTENTS Dago Section I. INTRODUCTION ' Purpose _ ^ Scope ' ^ Summary • g Section II. GENERAL INDEX y Section III. COUNTRY INDEX . Belgium France • P q -^ Germany 2U3 Italy 3-17 Japan 360 Soviet Union 388 Spain 429 Sweden . -

The Explosive Chemistry of Nitrogen* a Fascinating Journey from 9Th Century to the Present
GENERAL ARTICLE The Explosive Chemistry of Nitrogen* A Fascinating Journey From 9th Century to the Present Dheeraj Kumar and Anil J Elias The chemistry behind explosives is marked with the omnipres- ence of the element nitrogen. The discovery of the explosive properties of nitrogen-based compounds comprises many in- teresting and serendipitous observations made by inquisitive scientists. In this article, we unravel the fascinating history behind the development of explosives from the 9th century to the present. Every country with an army keeps on upgrading Dheeraj Kumar is an the sharpness and power of their arsenal, and in this regard, assistant professor at the the role played by the chemists in developing new explosives, Department of chemistry, Indian Institute of and the chemistry behind these explosives are explained in Technology Roorkee. He is this article. The basic chemical properties of explosives, their also recipient of “INSPIRE classification, comparison and methods of evaluation are ex- Faculty award" by plained. The current status of research in making new explo- Department of Science and Technology (DST). sives and the challenges involved in making explosive polyni- trogen compounds such as pentazolates and pentazenium are also illustrated. 1. Introduction Anil J. Elias is professor An interesting observation if one tries to analyse explosions from (HAG) at the Department of a chemist’s perspective is that the molecule, N2 is one of the ma- Chemistry, Indian Institute of jor products in almost all explosions except in a few cases in- Technology Delhi, New Delhi. He is also the recipient of the volving peroxides. This remarkable property of the element ni- ‘INSA Teacher Award’ by the trogen which otherwise is considered pretty inert is well known Indian National Science to chemists involved with the development of explosives and high Academy. -

Electronic Supplementary Information A
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is © The Royal Society of Chemistry 2018 Electronic Supplementary Information A Highly Emissive Fluorescent Zn-MOF: Molecular Decoding Strategies for Solvents and Trace Detection of Dunnite in Water Prasenjit Das and Sanjay K Mandal* Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Sector 81, Manauli PO, S.A.S. Nagar, Mohali, Punjab 140306, India Corresponding Author *E-mail: [email protected] S-1 Contents Item Description Page No Section-S1 General information – Materials and physical S-3 - S-6 measurements Section-S2 Synthesis of H4MTAIA and Zn-MOF (1) (Schemes 1 S-7 - S-8 and 2) Section-S3 Characterization of H4MTAIA (Fig. S1-S4) S-9 - S-11 (NMR, FTIR, HRMS and UV-Vis) Section-S4 Characterization of 1 (Fig. S5-S13) S-12 - S-18 ( FTIR, XRD, TGA, UV-Vis, Fluorescence) Section-S5 Gas/vapor sorption of 1 (Fig. S14-S18) S-19 - S-21 Section-S6 Fluorescence experiments and calculations S-22 - S-23 Section-S7 Solvent dependent fluorescence spectra of activated 1 S-24 - S-27 (Fig. S19-S24) Section-S8 Selective detection of Dunnite by activated 1 S-28 - S-34 (Scheme 3, Fig. S25-S36) Section-S9 Stern-Volmer plots (Fig. S37-S48) S-35 - S-40 Section-S10 Ammonium nitrate (AN) detection in water (Fig. S49- S-41 S50) Section-S11 Dunnite sensing in EtOH and DMF (Fig. S51-S54) S-42 - S-43 Section-S12 Calculation of detection limit (Fig. S55-S59) S-44 - S-47 Section-S13 Lifetime measurement and spectral overlap S-48 - S-49 (Fig. -

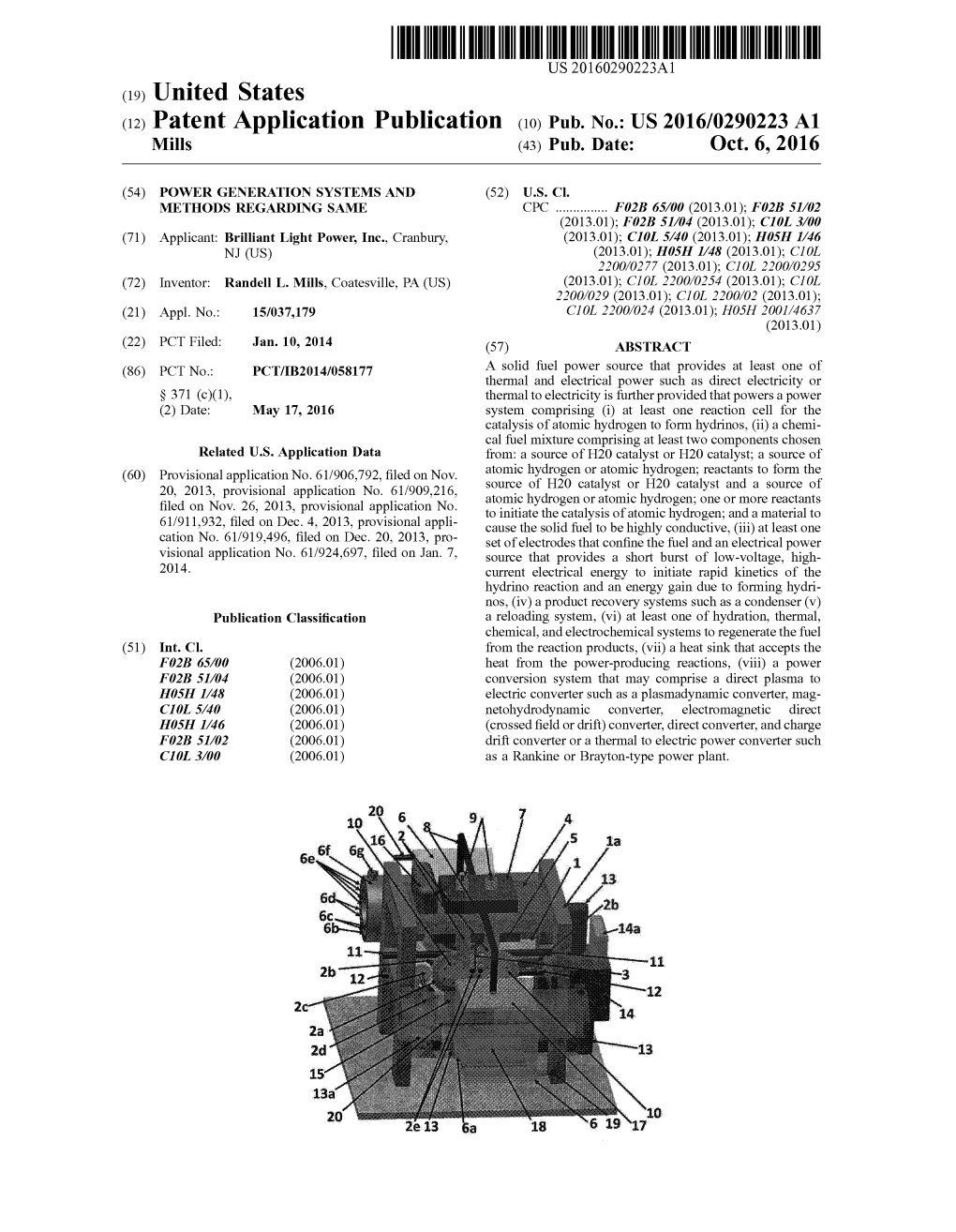
1 Power Generation Systems and Methods Regarding
POWER GENERATION SYSTEMS AND METHODS REGARDING SAME The present disclosure relates to the field of power generation and, in particular, to systems, devices, and methods for the generation of power. More specifically, embodiments of the present disclosure are directed to power generation devices and systems, as well as related methods, which produce plasma and thermal power and produces electrical power via a plasma to electric power converter or a thermal to electric power converter. In addition, embodiments of the present disclosure describe systems, devices, and methods that use the ignition of a water or water-based fuel source to generate mechanical power and/or thermal energy. Furthermore, the present disclosure is directed to electrochemical power systems that generate electrical power and/or thermal energy. These and other related embodiments are described in detail in the present disclosure. Power generation can take many forms, harnessing the power from plasma. Successful commercialization of plasma may depend on power generation systems capable of efficiently forming plasma and then capturing the power of the plasma produced. Plasma may be formed during ignition of certain fuels. These fuels can include water or water-based fuel source. During ignition, a plasma cloud of super-heated electron-stripped atoms is formed, and high-energy particles are ejected outwards. The highest energy particles ejected are the hydrogen ions that can transfer kinetic energy to a plasma to electric converter of the present disclosure. Power can also be generated through the use of a system or device that harnesses energy from the ignition of a fuel in a reaction vessel or combustion chamber. -

Chemical Hygiene Plan
Office of the Vice President for Research, Economic Development, and Knowledge Enterprise CHEMICAL HYGIENE PLAN THE UNIVERSITY OF TEXAS AT SAN ANTONIO OFFICE OF RESEARCH INTEGRITY LABORATORY SAFETY DIVISION 1 01-08-2019 REVIEW PAGE This original version of this procedure manual has been reviewed for regulatory compliance and best management practices by the undersigned individuals and is hereby adopted for use and compliance by all employees at all University of Texas at San Antonio owned or operated facilities. NAME TITLE DATE Amanda Haley Interim Laboratory Safety Manager and CHO 01-08-2019 Michelle Stevenson Associate Vice President of Research Administration 01-08-2019 Oleg Larionov Chair, Chemical Safety Committee 01-08-2019 Approved Rewrite: January 8, 2019 Replaces: May 17, 2011 Review Frequency: Annual This plan represents a significant rewrite. The January 8, 2019 version replaces the May 17, 2011 version. 2 01-08-2019 TABLE OF CONTENTS I. INTRODUCTION ........................................................................................................................................... 4 II. REGULATORY REQUIREMENTS .................................................................................................................... 5 III. RIGHTS AND RESPONSIBILITIES ................................................................................................................... 9 IV. TRAINING REQUIREMENTS ....................................................................................................................... -

Mcgraw-Hill Dictionary of Chemistry
McGraw-Hill Dictionary of Chemistry Second Edition McGraw-Hill New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto ebook_copyright 8.5 x 11.qxd 5/30/03 11:01 AM Page 1 Copyright © 2003 by The McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be repro- duced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-141797-4 The material in this eBook also appears in the print version of this title: 0-07-141046-5 All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. (“McGraw- Hill”) and its licensors reserve all rights in and to the work. Use of this work is subject to these terms.