Toward Refined Theoretical Models for the Description of Lipophilicity in Biomolecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
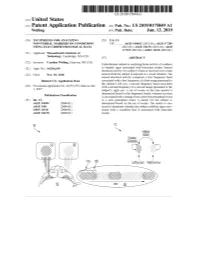
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

Sulphonmethane, Sulphonal, Diethylsulpho
is a combination of amylene hydrate and chloral hydrate, superior to the corresponding compounds of other elements. while chloralose, a combination of chloral hydrate and glucose, Experience has shown that, in the main, these claims were un- partakes of the action of morphin and is rather expensive. founded, though many, even now, claim that strontium bro- Chloretone, a more recent product, is not entirely devoid of mid disturbs the stomach less than the corresponding sodium danger and is not always so certain in its action as chloral or potassium salt. Another claim that is frequently made by hydrate, while butyl chloral hydrate, or crotón chloral hydrate, manufacturers of nostrums, like "Peacock's Bromides," is that is one of the older compounds that has been found wanting and they use "chemically pure" salts. Exactly what is meant by is now little used. Of the official compounds of this group we this claim is difficult to say, but the Pharmacopeia gives us a have: number of readily applied tests by which the salts themselves Chloralamid and Paraldehyd. may be tested. The manufacturers of nostrums, on the other Chlobalformamidum.—TJ. S.—Chloralformamid. Chlorala- hand, not infrequently add the very substances that are consid- mid. This has practically the same action as therapeutic ered contaminations. doses of chloral hydrate, the latter being formed in the body by {To be continued.) decomposition of chloralformamid. Average dose: 1 gm. (15 grains). Paraldehtdum.—TJ. S.—Paraldehyd is slower in its action transparent liquid, slower in its action than chloral hydrate, but also safer. It has the disadvantage of a persistently dis- A NEW NEEDLE HOLDER. -

31V March 1891 — Insomnia As a Disease Per Se and As a Symp¬
SYMPTOMATIC A' N D IDIOPATHIC INSOMNIA. ITS ETIOLOGY AND TREATMENT. B Y GEORGE DOUGLAS GRAY, M.B. 00O00O00O00 — 31V March 1891 — Insomnia as a disease per se and as a symp¬ tom of many diseases I have chosen as the subject of this thesis for several reasons. I have suffered . much from it myself and consequently have examined with more than ordinary clinical interest every case that has come under my notice, and in addition I have j found that, with the exception of odd notes in the medical journals, the literature on this subj.ect is comparatively rare. During this last half century since the introduction of steam power, and follov/lng it , the I "telegraph, electricity in its numerous forms, not to mention countless other time-saving devices, and with - an ever increasing population, competition or the race for life has become keener than at any " previoxis time, j -. : <■ , •- : 'V ■ " ■ ■ . - I The average human being lives at a very high pressure,! and this struggle for existence, with its concomitant 1 worries, has of late made Insomnia a much more fre¬ quent complaint than before. Indeed, in several of the older text-books on Practice of Physic (e.g. that of Dr. Hughes Bennet, published as lately as 1853) the subject is not even mentioned. In order to work well we must eat well and sleep well: loss of appet¬ ite and lowering of all bodily functions soon follow loss of sleep,therefore all the more important is it that the etiology and treatment of Insomnia should be thoroughly understood. -

Yasakli Maddeler
YASAKLI MADDELER Ülkemizde yarış atlarında kullanılan/kullanılma ihtimali olan ilaçların/maddelerin belirlenmesinde; ilacın farmakolojisi (öncelikle etkisi, etki grubu), farmasötik şekli, kullanılma yolu, kullanılma alanı, yarış sonucunu etkileme durumu, ulusal mevzuata göre veteriner hekimlikte ve/veya yarış atlarında kullanmak için ruhsatlı/izinli olup-olmama durumu, etiket-dışı kullanılma gibi hususlar dikkate alınmıştır. Eşik değeri (Threshold level) olan maddelerin (vücutta şekillenirler, çevre ve yem kaynaklı olabilirler) miktarı, belirlenen miktarı aştığında yasaklı-madde kullanılması/doping ihlali olarak değerlendirilir. Tedavide kullanılan/tarama limiti (Screening level) olan maddelerle ilgili değerlendirmede; bu maddelerle ilgili olarak yapılan doping analizlerinde ölçülen miktar, idrarda/plazmada belirlenen tarama değerini aştığında yasaklı madde kullanılması/doping-ihlali olarakdeğerlendirilir. Ana madde yanında, izomeri, metaboliti, metabolit izomeri, tuzları, esterleri, eterleri, diğer türevleri, ön-ilaç da yasaklı-madde listesinde yer alır; doping analizinde ortaya konulmaları doping ihlali olarak değerlendirilir. Maddeler ve yasaklı uygulamalar 6 başlık altında toplanmıştır. Bu liste ilgili uluslararası kuruluşların mevzuat ve düzenlemeleri ile ilgili maddelerin Farmakolojik özellikleri ve etkileri dikkate alınarak hazırlanmıştır. 1. YasaklıMaddeler Yasaklı maddeler; yarış atlarında tıbbi olarak kullanım yeri olmayan, atın yarış performansını açık şekilde etkileyen maddelerdir. İllegal kullanılan maddeler (Türkiye’de veteriner -

International Classification of Diseases
INTERNATIONAL CLASSIFICATION OF DISEASES MANUAL OF THE INTERNATIONAL STATISTICAL CLASSIFICATION OF DISEASES, INJURIES, AND CAUSES OF DEATH Based on the Recommendations of the Eighth Revision Conference, 1965, and Adopted by the Nineteenth World Health Assembly Volume 2 ALPHABETICAL INDEX WORLD HEALTH ORGANIZATION GENEVA 1969 Volume 1 Introduction List of Three-digit Categories Tabular List of Inclusions and Four-digit Sub- categories Medical Certification and Rules for Classification Special Lists for Tabulation Definitions and Recommendations Regulations Volume 2 Alphabetical Index PRINTED IN ENGLAND CONTENTS Introduction Page General arrangement of the Index ....................................... VIII Main sections ............................................................... VIII Structure ..................................................................... IX Code numbzrs .............................................................. x Primary and secondary conditions. ................................... x Multiple diagnoses. ........................................................ XI Spelling....................................................................... XI Order of listing ............................................................. Conventions used in the Index ........................................... XII Parentheses. ................................................................. XII Cross-referexes ........................................................... XI1 Abbreviation NEC. ...................................................... -

Detergents As Membrane-Mimetic Media for Structural Characterization of Membrane Proteins
Detergents as membrane-mimetic media for structural characterization of membrane proteins. by David Vincent Tulumello A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Biochemistry University of Toronto © Copyright by David Vincent Tulumello 2012 Detergents as membrane-mimetic media for structural characterization of membrane proteins. David Vincent Tulumello Doctor of Philosophy Department of Biochemistry University of Toronto 2012 Abstract Membrane proteins are essential cellular components, responsible for a wide variety of biological functions. In order to better understand such aspects of cell activity, researchers have pursued detailed structural analysis of this class of proteins. Because of the complexities in isolating and studying membrane proteins in their native environment, detergents are often employed as a membrane mimetic media. This thesis examines several features of transmembrane (TM) protein structure and folding in detergents through which we are able to gain insights into membrane protein folding, as well as explore the suitability of detergents as membrane-mimetic environments. We first compare the helix-helix association of a series of model TM sequences in a native bilayer to the corresponding association in a detergent environment. We find that while various classes of helix-helix interaction motifs are preserved in detergents, alterations in detergent solvation may, in turn, lead to altered association affinity. We further explore this phenomenon through investigation of the consequences of the insertion of a strongly polar residue into a TM segment. In these studies we find a correlation between sequence-dependent ii alterations in detergent solvation and predicted in vivo folding. We also extend such analyses to a variety of detergents and native TM segments, finding that native secondary structure, as it occurs in the context of a full-length protein, is generally well preserved in a variety of detergents. -

Federal Register Volume 31 • Number 182
FEDERAL REGISTER VOLUME 31 • NUMBER 182 Tuesday, September 20, 1966 • Washington, D.C. Pages 12427-12470 Agencies in this issue—• Atomic Energy Commission Civil Aeronautics Board Civil Service Commission Comptroller of the Currency Consumer and Marketing Service Defense Department Engineers Corps Federal Aviation Agency Federal Maritime Commission Federal Power Commission Federal Trade Commission Fish and Wildlife Service Food and Drug Administration General Services Administration International Commerce Bureau Interstate Commerce Commission Land Management Bureau National Bureau of Standards Post Office Department Reclamation Bureau Securities and Exchange Commission Wage and Hour Division Detailed list of Contents appears- inside. Latest Edition Guide to Record Retention Requirements [Revised as of January 1, 1966] This useful reference tool is designed keep them, and (3) how long they to keep industry and the general must be kept. Each digest also public informed concerning published includes a reference to the full text requirements in laws and regulations of the basic law or regulation govern relating to records-retention. It corn- ing such retention. tains over 900 digests detailing the retention periods for the many types of records required to be kept under The booklet’s index, numbering over Federal laws and rules. 2,000 items, lists for ready reference the categories of persons, companies, The “Guide” tells the user (1) what and products affected by Federal records must be kept, (2) who must record-retention requirements. Price; 40 cents Compiled by Office of the Federal Register, Nati onal Archives and Records Service, General Services Admi nistration * Order from Superintendent of Documents, U.S. Government Printing Office Washington, D.C. -

Sider Its Chemical Aspects ; and (3) to Discuss Its Therapeutic Said to Have Already Been Arrived At
252 DR. SAINSBURY: CHEMICAL CONSTITUTION AND THERAPEUTIC ACTION. was instituted ; and it is to be particularly noted that of SummingS up the evidence, so far as it is possible to sum up the total 17 cases 13 occurred in the period ending 1876. oon a subject as to which so much is still to be learned, we In the various streets within a quarter-mile radius of the seems to reach the general conclusion that, as a result of the hospital the percentage of the population attacked in the simultaneouss action of causes favourable to the spread of ten,years 1871-80 ranged from 4 per cent. to 25 per cent., infection,ii the contagion of small-pox may sometimes be con- the average being 9 per cent.2 In the workhouse, on the veyedv atmospherically to a distance much greater than had other hand, only some 3 per cent. were attacked. The tbeen usually admitted-a distance measurable by quarters of difference is certainly considerable; but the fact that the miles.r In endeavouring to summarise the agencies which cases in the workhouse practically stopped after 1876, 1:have to do with this result it is necessary to bear in mind when revaccination became part of the administrative thatt other unknown agencies may also be involved, and that system, goes far to account for the low attack-rate calcu- sometimess the joint action of all the known agencies may not lated over the whole eleven years. Whether, in addition, bet necessary, special activity of some, perhaps, atoning for anything (is to be attributed to the age-distribution of relativer deficiency or absence, and vice versâ, of others the workhouse inmates or to previous small-pox or to Keeping1 this in view, the factors whose coincident operation the well - regulated conditions as to ventilation, clean- (can produce the result in question may be subdivided as liness, &c. -

Letter to the Editor: a Response to Horne and Lucey (2017)
Carver, J. A., Thorn, D. C., Ecroyd, H. and Holt, C. (2017) Letter to the Editor: a response to Horne and Lucey (2017). Journal of Dairy Science, 100(7), pp. 5121-5124. There may be differences between this version and the published version. You are advised to consult the publisher’s version if you wish to cite from it. http://eprints.gla.ac.uk/143198/ Deposited on: 7 July 2017 Enlighten – Research publications by members of the University of Glasgow http://eprints.gla.ac.uk Response to Horne and Lucey In 2013, we wrote a review in the Journal of Dairy Science (Holt et al., 2013) and another in the International Dairy Journal in 2015 (Thorn et al., 2015), both dealing with the application of modern protein science concepts to the chemistry and physics of caseins. These were substantial documents with an important message. We brought together results from the burgeoning new fields of intrinsically disordered proteins (IDPs), amyloid fibril formation and molecular chaperone action to provide a radically new view of caseins and the casein micelle. We showed, through various examples, that caseins were far from unique among proteins in their behaviour and that other IDPs provided better models for understanding caseins than either globular proteins or soap molecules. New concepts have been developed within the IDP field to explain how IDPs interact with each other and with intermediately folded globular proteins to form either amyloid fibrils, amorphous aggregates, gels or precipitates. These ideas relate to interactions between main-chain groups having low sequence specificity (also called promiscuous interactions) that are important in the action of caseins as molecular chaperones and in forming amorphous aggregates such as the casein micelle. -

TEXAS RACING COMMISSION January 28, 2021 To
TEXAS RACING COMMISSION P. O. Box 12080, Austin, Texas 78711-2080 8505 Cross Park Drive, Suite 110, Austin, Texas 78754-4552 Phone (512) 833-6699 Fax (512) 833-6907 www.txrc.texas.gov January 28, 2021 To: Stewards, Commission Veterinarians, Test Barn Supervisors, Practicing Veterinarians, Owners, and Trainers From: Chuck Trout, Executive Director Re: Effective February 25, 2021 changes to the following documents: • Permissible Levels of Therapeutic Medications and Naturally Occurring Substances • Equine Medication Classification Policy and Penalty Guidelines • Equine Medication Classification List. This memo is to provide notice that the above listed documents are to be replaced effective this date. The changes include, but are not limited to: • Changes to the list of Permissible Levels of Therapeutic Medications and Naturally Occurring Substances; • Changes to the Equine Medication Classification Policy and Penalty Guidelines; • Changes to the Equine Medication Classification List. These documents are subject to further revision at any time. Test Barn Supervisors - please post this memo and the revised documents in the test barn as soon as possible. Also, please distribute copies of the Permissible Levels of Therapeutic Medications and Naturally Occurring Substances and Equine Medication Classification List to the practicing veterinarians at your racetrack. Licensing Staff - please post this memo and the revised documents where they may be viewed by the public as soon as possible. Copies of these documents will be made available on the Commission's website at http://www.txrc.texas.gov. Attachments: Permissible Levels of Therapeutic Medications and Naturally Occurring Substances Equine Medication Classification Policy and Penalty Guidelines Equine Medication Classification List TEXAS RACING COMMISSION P. -

Time-Certainly for Months, Often for Many Years-Before Admitted to the Metropolitan Hospital, Under the Care of Mr
1105 from the hydatid cyst extends usually over a long period of In June, 1906, a married woman, aged 22 years, was time-certainly for months, often for many years-before admitted to the Metropolitan Hospital, under the care of Mr. the patient comes under observation, and the capacity of the W. D. Harmer, for a severe attack of abdominal pain with human organism for reaction to the hydatid products may vomiting and constipation. The vomited material was deeply thus become exhausted. Moreover, the absorption of hydatid bile-stained but never fsecal. It was noted that the urine products may often be inadequate, and more often still it is was claret-coloured and that it contained a few blood cells, probably irregular both in amount and in time. On these though not nearly enough to account for the colour. A few grounds alone it is only reasonable to expect that the pre- granular and epithelial casts were present ; there was no cipitable content of hydatid antisera from the human subject bile or pus. No spectroscopic examination of the urine was should inevitably be small in the majority of cases, and made. Mr. Harmer decided that the patient was not suffer- it is not surprising that in some cases it should be in- ing from intestinal obstruction, so large enemata were appreciable. given, the patient being placed on milk diet. There was In regard to the hydatid fluid we must note (a) that not no improvement in her condition until the sixth day after all hydatid fluids are equally capable of interacting with admission, when the vomiting stopped and the bowels became hydatid antisera to give a precipitate, and that some appear fairly regular. -

Side-Chain Hydrophobicity Scale Derived from Transmembrane Protein Folding Into Lipid Bilayers
Side-chain hydrophobicity scale derived from transmembrane protein folding into lipid bilayers C. Preston Moon and Karen G. Fleming1 Thomas C. Jenkins Department of Biophysics, Johns Hopkins University, 3400 North Charles Street, Baltimore, MD 21218 Edited* by Christopher Miller, Brandeis University, Waltham, MA, and approved April 29, 2011 (received for review March 11, 2011) The transfer free energies of the twenty natural amino acid Results and Discussion side chains from water to phospholipid bilayers make a major con- We used the outer membrane phospholipase A (OmpLA) as a tribution to the assembly and function of membrane proteins. transmembrane scaffold on which to introduce amino acid side Measurements of those transfer free energies will facilitate the chains of our choice at various membrane depths. We selected identification of membrane protein sequences and aid in the under- OmpLA because it: (a) spontaneously folds and inserts into lipid standing of how proteins interact with membranes during key bio- membranes from a solubilized unfolded state (14), (b) has a logical events. We report the first water-to-bilayer transfer free known three-dimensional structure (Fig. 1A) (15), and (c) has energy scale (i.e., a “hydrophobicity scale”) for the twenty natural enzymatic activity that can be monitored to confirm native-like amino acid side chains measured in the context of a native trans- folding (16, 17). To measure side-chain hydrophobicity in a mem- ’ membrane protein and a phospholipid bilayer. Our measurements brane, we selected the alanine at position 210 in OmpLAs reveal parity for apolar side-chain contributions between soluble sequence as a host site for substitution to each of the other 19 and membrane proteins and further demonstrate that an arginine amino acids as guests.