Methyltransferase Nsp16/Nsp10 Complex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigator Initiated Study IRB-29839 an Open-Label Pilot Study To
Investigator Initiated Study IRB-29839 An open-label pilot study to evaluate the efficacy and safety of a combination treatment of Sonidegib and BKM120 for the treatment of advanced basal cell carcinomas Version 05 September 2016 NCT02303041 DATE: 12Dec2018 1 Coordinating Center Stanford Cancer Center 875 Blake Wilbur Drive Stanford, CA 94305 And 450 Broadway, MC 5334 Redwood City, CA 94603 Protocol Director and Principal Investigator Anne Lynn S Chang, MD, Director of Dermatological Clinical Trials 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Co-Investigator Anthony Oro, MD PhD 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Biostatistician Shufeng Li, MS 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Study Coordinator Ann Moffat 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] 2 Table of Contents 1 Background ................................................................. 7 1.1 Disease Background ..................................................... 7 1.2 Hedgehog Pathway and mechanism of action ............................... 7 1.3 PI3K Pathway and mechanism of action ................................... 9 1.4 Sonidegib Compound Information ............ Error! Bookmark not defined. 1.4.1 Preclinical Studies for Sonidegib ....................................................................11 1.4.2 Muscular system...............................................................................................13 1.4.3 Skeletal system ................................................................................................13 -

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

Maintenance Drug List
Commonly Prescribed Maintenance Medications Maintenance (or long-term) medications are those dosage form may be used to treat more than one drugs you may take on a regular basis to treat conditions medical condition. In these cases, each medication may such as high cholesterol, high blood pressure or diabetes. be classified according to its first U.S. Food and Drug Based on your benefit plan, you may get up to a 90-day Administration (FDA) approved use. Some strengths supply of covered maintenance medication delivered and/or formulations listed may not be covered. to you through a home delivery program or at select Please note that oral contraceptives are maintenance retail pharmacies. medications and are part of this list. However, this Some plans may require you to fill these prescriptions list does not include the trade names of all available through home delivery or at select retail pharmacies oral contraceptives because there are a large number in order to receive coverage. of products. Commonly used maintenance medications are listed in Coverage is always subject to the terms and limits of this guide. This list is not all-inclusive and may change your benefit plan. Please verify with your plan if there from time to time. are any additional requirements before a drug may be Most generic drugs listed are followed by a reference covered. For details about your plan, check your benefit brand drug in (parentheses). The brand name drug materials or call the number on your member ID card. in parentheses is listed for reference and may not be Third-party brand names are the property of their covered under your benefit. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -
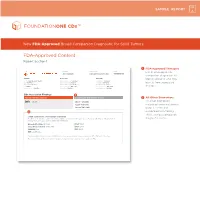
FDA-Approved Content Report Section 1
SAMPLE REPORT New FDA-Approved Broad Companion Diagnostic for Solid Tumors FDA-Approved Content Report Section 1 1 FDA-Approved Therapies PATIENT TUMOR TYPE TRF# List of FDA-approved Jane Sample Lung adenocarcinoma TRFXXXXXX companion diagnostics to PATIENT PHYSICIAN SPECIMEN identify patients who may DISEASE Lung adenocarcinoma ORDERING PHYSICIAN Not Given SPECIMEN SITE Not Given NAME Not Given MEDICAL FACILITY Not Given SPECIMEN ID Not Given benefi t from associated DATE OF BIRTH Not Given ADDITIONAL RECIPIENT Not Given SPECIMEN TYPE Not Given SEX Female MEDICAL FACILITY ID Not Given DATE OF COLLECTION Not Given therapies MEDICAL RECORD # Not Given PATHOLOGIST Not Given SPECIMEN RECEIVED Not Given CDx Associated Findings 1 GENOMIC FINDINGS DETECTED FDA-APPROVED THERAPEUTIC OPTIONS 2 All Other Biomarkers EGFR L858R Gilotrif® (Afatinib) All other biomarkers, Iressa® (Gefitinib) including tumor mutational Tarceva® (Erlotinib) burden (TMB) and 2 microsatellite instability (MSI), without companion OTHER ALTERATIONS & BIOMARKERS IDENTIFIED Results reported in this section are not prescriptive or conclusive for labeled use of any specific therapeutic product. See diagnostic claims professional services section for additional information. Microsatellite Status MS-Stable PTCH1 T416S Tumor Mutation Burden 11 Muts/Mb RBM10 Q494* CDKN2A/B loss TP53 R267P EGFR amplification § Refer to appendix for limitation statements related to detection of any copy number alterations, gene rearrangements, MSI or TMB result in this section. Please refer to appendix -

Lifitegrast for the Treatment of Dry Eye Disease in Adults
Expert Opinion on Pharmacotherapy ISSN: 1465-6566 (Print) 1744-7666 (Online) Journal homepage: http://www.tandfonline.com/loi/ieop20 Lifitegrast for the treatment of dry eye disease in adults Eric D. Donnenfeld, Henry D. Perry, Alanna S. Nattis & Eric D. Rosenberg To cite this article: Eric D. Donnenfeld, Henry D. Perry, Alanna S. Nattis & Eric D. Rosenberg (2017): Lifitegrast for the treatment of dry eye disease in adults, Expert Opinion on Pharmacotherapy, DOI: 10.1080/14656566.2017.1372748 To link to this article: http://dx.doi.org/10.1080/14656566.2017.1372748 Accepted author version posted online: 25 Aug 2017. Published online: 04 Sep 2017. Submit your article to this journal Article views: 11 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ieop20 Download by: [108.6.184.233] Date: 04 September 2017, At: 04:29 EXPERT OPINION ON PHARMACOTHERAPY, 2017 https://doi.org/10.1080/14656566.2017.1372748 DRUG EVALUATION Lifitegrast for the treatment of dry eye disease in adults Eric D. Donnenfelda, Henry D. Perryb, Alanna S. Nattisb and Eric D. Rosenbergc aOphthalmic Consultants of Long Island, New York University Medical Center, Garden City, NY, USA; bOphthalmic Consultants of Long Island, Nassau University Medical Center, Rockville Centre, NY, USA; cWestchester Medical Center, Valhalla, NY, USA ABSTRACT ARTICLE HISTORY Introduction: Dry eye disease (DED) is a common ocular disorder that can have a substantial burden on Received 14 June 2017 quality of life and daily activities. Lifitegrast ophthalmic solution 5.0% is the first medication approved in Accepted 24 August 2017 the US for the treatment of the signs and symptoms of DED. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y
Published OnlineFirst November 6, 2015; DOI: 10.1158/1078-0432.CCR-15-1588 Clinical Trial Brief Report Clinical Cancer Research An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y. Sarin, Anthony E. Oro, and Anne Lynn S. Chang Abstract Purpose: To assess the tumor response to the smoothened sive disease with sonidegib. Three patients experienced stable (SMO) inhibitor, sonidegib (LDE225), in patients with an disease and discontinued sonidegib either due to adverse events advanced basal cell carcinoma (BCC) resistant to treatment with (n ¼ 1) or due to election for surgery (n ¼ 2). The response of one vismodegib (GDC0449). patient was not evaluable. SMO mutations with in vitro data Experimental Design: Nine patients with an advanced suggesting resistance to Hh pathway inhibition were identified BCC that was previously resistant to treatment with vismode- in 5 patients, and none of these patients experienced responses gib were given sonidegib in this investigational, open- while on sonidegib. label study. Tumor response was determined using the Conclusion: Patients with advanced BCCs that were response evaluation criteria in solid tumors. SMO mutations previously resistant to treatment with vismodegib similarly were identified using biopsy samples from the target BCC demonstrated treatment resistance with sonidegib. Patients location. who have developed treatment resistance to an SMO inhibitor Results: The median duration of treatment with sonidegib was may continue to experience tumor progression in response to 6 weeks (range, 3–58 weeks). Five patients experienced progres- other SMO inhibitors. Clin Cancer Res; 1–5. Ó2015 AACR. Introduction Sonidegib (LDE225) is a new SMO inhibitor approved in 2015 by the FDA for locally advanced BCCs. -

Summary of Appeals & Independent Review Organization
All Other Appeals All other appeals are for drugs not in an inpatient hospital setting that Molina was not able to approve. Sometimes, the clinical information sent to us for these drugs do not meet medical necessity on initial review. When drug preauthorization requests are denied, a member or provider has the right to appeal. Appeals allow time to provide more clinical information. With complete clinical information, we can usually approve the drug. These are considered an appeal overturn. When the denial decision is not overturned, it is considered upheld. Service Code/Drug Name Service Code Description Number of Appeals Number of Appeals Total Appeals Upheld Overturned A9274 EXTERNAL AMB INSULIN DEL SYSTEM DISPOSABLE EA 0 1 1 Abatacept 3 1 4 Abemaciclib 1 0 1 Acalabrutinib 0 1 1 Acne Combination - Two Ingredient 1 0 1 Acyclovir 0 1 1 Adalimumab 7 14 21 Adrenergic Combination - Two Ingredient 1 0 1 Aflibercept 0 2 2 Agalsidase 1 0 1 Alfuzosin 1 0 1 Amantadine 1 0 1 Ambrisentan 0 1 1 Amphetamine 0 1 1 Amphetamine Mixtures - Two Ingredient 1 8 9 Apixaban 7 21 28 Apremilast 12 13 25 Aprepitant 0 1 1 Aripiprazole 5 9 14 ARNI-Angiotensin II Recept Antag Comb - Two Ingredient 6 9 15 Asenapine 0 1 1 Atomoxetine 1 3 4 Atorvastatin 0 1 1 Atovaquone 1 0 1 Axitinib 0 1 1 Azathioprine 0 1 1 Azilsartan 1 0 1 Azithromycin 1 0 1 Baclofen 0 1 1 Baricitinib 1 1 2 Belimumab 0 1 1 Benralizumab 1 0 1 Beta-blockers - Ophthalmic Combination - Two Ingredient 0 2 2 Bimatoprost 0 1 1 Botulinum Toxin 1 4 5 Buprenorphine 4 3 7 Calcifediol 1 0 1 Calcipotriene -

Xiidra, INN-Lifitegrast
EMA/334174/2020 Committee for Medicinal Products for Human Use (CHMP) Withdrawal assessment report Xiidra International non-proprietary name: lifitegrast Procedure No. EMEA/H/C/004653/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Classified as confidential by the European Medicines Agency Table of Contents 1. Joint Rapporteur’s Recommendations ..................................................... 5 2. Executive summary ................................................................................. 7 2.1. Problem statement ............................................................................................... 7 2.1.1. Disease or condition ........................................................................................... 7 2.1.2. Epidemiology and risk factors, screening tools/prevention ...................................... 7 2.1.3. Aetiology and pathogenesis ................................................................................ 7 2.1.4. Clinical presentation ........................................................................................... 8 2.1.5. Management .................................................................................................... -

P&T Summary 1Q2021
BLUE SHIELD OF CALIFORNIA FIRST QUARTER 2021 FORMULARY AND MEDICATION POLICY UPDATES EFFECTIVE MARCH 3, 2021 for Large Group, Small Group, and Individual & Family Plans The Blue Shield of California (BSC) Pharmacy and Therapeutics (P&T) Committee, consisting of network physicians and pharmacists, regularly evaluates drugs for formulary inclusion and medication policy coverage criteria. The first quarter 2021 P&T Committee decisions on formulary changes and medication policy changes, which apply to Commercial members with an outpatient drug benefit, are summarized below: PHARMACY BENEFIT FORMULARY UPDATE: Please refer to the appropriate drug formulary posted on our website for the following information: • Quantity limits, if applicable, for specific drugs • Formulary status of newly available strengths of existing drugs. Note: The formulary status of newly available strengths of existing drugs will have the same formulary status as the existing strengths unless otherwise noted. • Non-formulary and non-preferred generic drugs that do not require prior authorization or step therapy • Brand-name medications that are now non-formulary or non-preferred because these medications have newly available generic equivalents covered on the formulary Formularies are available at blueshieldca.com/pharmacy. Select the appropriate drug formulary – “Standard Drug Formulary”, “Value Drug Formulary”, or “Plus Drug Formulary”. Summary of changes to the Medicare formularies are available at blueshieldca.com/pharmacy. Select “Medicare Drug Formulary”, then -

Imatinib (Gleevec™)
Biologicals What Are They? When Did All of this Happen? There are Clear Benefits. Are there also Risks? Brian J Ward Research Institute of the McGill University Health Centre Global Health, Immunity & Infectious Diseases Grand Rounds – March 2016 Biologicals Biological therapy involves the use of living organisms, substances derived from living organisms, or laboratory-produced versions of such substances to treat disease. National Cancer Institute (USA) What Effects Do Steroids Have on Immune Responses? This is your immune system on high dose steroids projects.accessatlanta.com • Suppress innate and adaptive responses • Shut down inflammatory responses in progress • Effects on neutrophils, macrophages & lymphocytes • Few problems because use typically short-term Virtually Every Cell and Pathway in Immune System ‘Target-able’ (Influenza Vaccination) Reed SG et al. Nature Medicine 2013 Nakaya HI et al. Nature Immunology 2011 Landscape - 2013 Antisense (30) Cell therapy (69) Gene Therapy (46) Monoclonal Antibodies (308) Recombinant Proteins (93) Vaccines (250) Other (81) http://www.phrma.org/sites/default/files/pdf/biologicsoverview2013.pdf Therapeutic Category Drugs versus Biologics Patented Ibuprofen (Advil™) Generic Ibuprofen BioSimilars/BioSuperiors ? www.drugbank.ca Patented Etanercept (Enbrel™) BioSimilar Etanercept Etacept™ (India) Biologics in Cancer Therapy Therapeutic Categories • Hormonal Therapy • Monoclonal antibodies • Cytokine therapy • Classical vaccine strategies • Adoptive T-cell or dendritic cells transfer • Oncolytic