Extreme Physiological Adaptations As Predictors of Climatechange
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomic Status of the Genus Sotalia: Species Level Ranking for “Tucuxi” (Sotalia Fluviatilis) and “Costero” (Sotalia Guianensis) Dolphins
MARINE MAMMAL SCIENCE, **(*): ***–*** (*** 2007) C 2007 by the Society for Marine Mammalogy DOI: 10.1111/j.1748-7692.2007.00110.x TAXONOMIC STATUS OF THE GENUS SOTALIA: SPECIES LEVEL RANKING FOR “TUCUXI” (SOTALIA FLUVIATILIS) AND “COSTERO” (SOTALIA GUIANENSIS) DOLPHINS S. CABALLERO Laboratory of Molecular Ecology and Evolution, School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland, New Zealand and Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia F. TRUJILLO Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia J. A. VIANNA Sala L3–244, Departamento de Biologia Geral, ICB, Universidad Federal de Minas Gerais, Avenida Antonio Carlos, 6627 C. P. 486, 31270–010 Belo Horizonte, Brazil and Escuela de Medicina Veterinaria, Facultad de Ecologia y Recursos Naturales, Universidad Andres Bello Republica 252, Santigo, Chile H. BARRIOS-GARRIDO Laboratorio de Sistematica´ de Invertebrados Acuaticos´ (LASIA), Postgrado en Ciencias Biologicas,´ Facultad Experimental de Ciencias,Universidad del Zulia, Avenida Universidad con prolongacion´ Avenida 5 de Julio, Sector Grano de Oro, Maracaibo, Venezuela M. G. MONTIEL Laboratorio de Ecologıa´ y Genetica´ de Poblaciones, Centro de Ecologıa,´ Instituto Venezolano de Investigaciones Cientıficas´ (IVIC), San Antonio de los Altos, Carretera Panamericana km 11, Altos de Pipe, Estado Miranda, Venezuela S. BELTRAN´ -PEDREROS Laboratorio de Zoologia,´ Colec¸ao˜ Zoologica´ Paulo Burheim, Centro Universitario´ Luterano de Manaus, Manaus, Brazil 1 2 MARINE MAMMAL SCIENCE, VOL. **, NO. **, 2007 M. MARMONTEL Sociedade Civil Mamiraua,´ Rua Augusto Correa No.1 Campus do Guama,´ Setor Professional, Guama,´ C. P. 8600, 66075–110 Belem,´ Brazil M. C. SANTOS Projeto Atlantis/Instituto de Biologia da Conservac¸ao,˜ Laboratorio´ de Biologia da Conservac¸ao˜ de Cetaceos,´ Departamento de Zoologia, Universidade Estadual Paulista (UNESP), Campus Rio Claro, Sao˜ Paulo, Brazil M. -

Morphometrics of the Dolphin Genus Lagenorhynchus: Deciphering A
Morphometrics of the dolphin genus Lagenorhynchus: deciphering a contested phylogeny Allison Galezo1,2 and Nicole Vollmer1,3 1 Department of Vertebrate Zoology, Smithsonian Institution National Museum of Natural History 2 Department of Biology, Georgetown University 3 NOAA National Systematics Laboratory Background Results & Analysis Discussion • Our morphological data support the hypothesis that Figure 1. Phenogram from cluster analysis of dolphin skull Figure 2. Species symbols key with sample sizes. measurements. Calculated using Euclidian distances and the genus Lagenorhynchus is not monophyletic, evident Height l La. acutus (24) p C. commersonii (5) a b c Ward’s method. from the separation in the phenogram of La. albirostris Height p C. eutropia (2) Recent phylogenetic studies1-7 have indicated that the Distance l La. albirostris (10) and La. acutus from the other Lagenorhynchus species, 0 2 4 6 8 p genus Lagenorhynchus, currently containing the species 0 2 4 6 8 l La. australis (7) C. heavisidii (1) u Li. borealis (11) and the mix of genera in the lowermost clade (Figure 1). L. obliquidensa, L. acutusb , L. albirostrisc, L. obscurusd , L. l La. obliquidens (28) p C. hectori (2) n Unknown (1) • Our results show that La. obscurus and La. obliquidens e f cruciger , and L. australis , is not monophyletic. These C. commersonii l La. obscurus (15) are very similar morphologically, which supports the C. commersonii species were originally grouped together because of C.C. cocommemmersoniirsonii C. commeC. hersoniictori hypothesis that they are closely related: they have C. commeC. hersoniictori similarities in external morphology and coloration, but C. commeC. hersoniictori Figure 3. -
Global Patterns in Marine Mammal Distributions
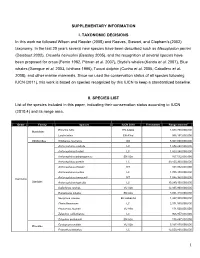
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

SHORT-FINNED PILOT WHALE (Globicephala Macrorhynchus): Western North Atlantic Stock
February 2019 SHORT-FINNED PILOT WHALE (Globicephala macrorhynchus): Western North Atlantic Stock STOCK DEFINITION AND GEOGRAPHIC RANGE There are two species of pilot whales in the western North Atlantic - the long-finned pilot whale, Globicephala melas melas, and the short-finned pilot whale, G. macrorhynchus. These species are difficult to differentiate at sea and cannot be reliably visually identified during either abundance surveys or observations of fishery mortality without high-quality photographs (Rone and Pace 2012); therefore, the ability to separately assess the two species in U.S. Atlantic waters is complex and requires additional information on seasonal spatial distribution. Pilot whales (Globicephala sp.) in the western North Atlantic occur primarily along the continental shelf break from Florida to the Nova Scotia Shelf (Mullin and Fulling 2003). Long-finned and short- finned pilot whales overlap spatially along the mid-Atlantic shelf break between Delaware and the southern flank of Georges Bank (Payne and Heinemann 1993; Rone and Pace 2012). Long-finned pilot whales have occasionally been observed stranded as far south as South Carolina, and short- finned pilot whales have occasionally been observed stranded as far north as Massachusetts (Pugliares et al. 2016). The exact latitudinal ranges of the two species remain uncertain. However, south of Cape Hatteras most pilot whale sightings are expected to be short- Figure 1. Distribution of long-finned (open symbols), short-finned finned pilot whales, while north of (black symbols), and possibly mixed (gray symbols; could be ~42°N most pilot whale sightings are either species) pilot whale sightings from NEFSC and SEFSC expected to be long-finned pilot whales shipboard and aerial surveys during the summers of 1998, 1999, (Figure 1; Garrison and Rosel 2017). -

Riverine and Marine Ecotypes of Sotalia Dolphins Are Different Species
Marine Biology (2005) 148: 449–457 DOI 10.1007/s00227-005-0078-2 RESEARCH ARTICLE H.A. Cunha Æ V.M.F. da Silva Æ J. Lailson-Brito Jr M.C.O. Santos Æ P.A.C. Flores Æ A.R. Martin A.F. Azevedo Æ A.B.L. Fragoso Æ R.C. Zanelatto A.M. Sole´-Cava Riverine and marine ecotypes of Sotalia dolphins are different species Received: 24 December 2004 / Accepted: 14 June 2005 / Published online: 6 September 2005 Ó Springer-Verlag 2005 Abstract The current taxonomic status of Sotalia species cific status of S. fluviatilis ecotypes and their population is uncertain. The genus once comprised five species, but structure along the Brazilian coast. Nested-clade (NCA), in the twentieth century they were grouped into two phylogenetic analyses and analysis of molecular variance (riverine Sotalia fluviatilis and marine Sotalia guianensis) of control region sequences showed that marine and that later were further lumped into a single species riverine ecotypes form very divergent monophyletic (S. fluviatilis), with marine and riverine ecotypes. This groups (2.5% sequence divergence; 75% of total molec- uncertainty hampers the assessment of potential impacts ular variance found between them), which have been on populations and the design of effective conservation evolving independently since an old allopatric fragmen- measures. We used mitochondrial DNA control region tation event. This result is also corroborated by cyto- and cytochrome b sequence data to investigate the spe- chrome b sequence data, for which marine and riverine specimens are fixed for haplotypes that differ by 28 (out Communicated by J. P. -

Distribution and Movement Patterns of Killer Whales (Orcinus Orca) in the Northwest Atlantic. ICES CM 2008/B:20
ICES CM 2008/B:20 Distribution and Movement Patterns of Killer Whales (Orcinus orca) in the Northwest Atlantic Tara S. Stevens1,2 and Jack W. Lawson1 (1) Fisheries and Oceans Canada, NAFC, 80 East White Hills Road, St. John’s, Newfoundland and Labrador A1C 5X1, Canada (2) Memorial University of Newfoundland, Cognitive and Behavioural Ecology Program, St. John’s, Newfoundland and Labrador A1B 3X7, Canada Corresponding author e-mail: [email protected] ABSTRACT: Killer whales (Orcinus orca) occur throughout the northwest Atlantic. A sightings database and photographic catalogue, created mostly from opportunistic sources, is used to examine the occurrence of killer whales in Atlantic Canada. A majority of the sightings are from the Newfoundland and Labrador Region despite comparable observer coverage in adjacent areas such as the Gulf of St. Lawrence and Scotian Shelf, which suggests greater abundance in and habitat preference for Newfoundland and Labrador waters. Killer whales occur in all months of the year and in both near- and offshore regions, although particular sighting patterns may represent local observer effort and awareness. The distribution, movement, and residency patterns of killer whales may be closely linked to that of their prey; they have been observed harassing, attacking, and eating marine mammals, including minke whales (Balaenoptera acuterostrata), dolphins, and seals, and potentially eating fish. Some killer whales appear to remain year round in the Newfoundland and Labrador area and have been sighted during the spring within pack ice, potentially in association with breeding harp seals (Phoca groenlandica). Based on photographic records, individual killer whales in this area have been shown to move hundreds of kilometers within a year. -

Lagenorhynchus Obscurus – Dusky Dolphin
Lagenorhynchus obscurus – Dusky Dolphin to be split in due course (LeDuc et al. 1999; Harlin- Cognato 2010). Several recent phylogenies have shown that L. obscurus consistently groups with L. obliquidens (Pacific White-sided Dolphin) and separately to L. australis (Peale’s Dolphin) and L. cruciger (Hourglass Dolphin). The two North Atlantic members of the genus (L. albirostris, the White-beaked Dolphin, and L. acutus, the Atlantic White-sided Dolphin) appear distinct from all other members of the genus. Current thinking would either split L. obscurus and L obliquidens into the genus Sagmatius (LeDuc et al. 1999; May-Collado & Agnarsson 2006) or make it an entirely new genus (Harlin-Cognato 2010). Sara Golaski / Namibian Dolphin Project Three subspecies of Dusky Dolphin are generally suggested based on both molecular (Harlin-Cognato et al. Regional Red List status (2016) Least Concern*† 2007) and morphological (Van Waerebeek 1993a, 1993b) differences: L. o. obscurus in southern Africa, L. o. fitzroyi National Red List status (2004) Data Deficient in southern South America, and an un-named subspecies Reasons for change Non-genuine change: in New Zealand (Perrin 2002). Subspecies classification New information awaits formal description (Cipriano & Webber 2010). Global Red List status (2008) Data Deficient TOPS listing (NEMBA) (2007) None Assessment Rationale Large schools and frequent sightings of Dusky Dolphins in CITES listing (2003) Appendix II both the northern (Namibia) and southern (South Africa) Endemic No Benguela suggest high abundance and more than 10,000 mature individuals. No major threats were identified, *Watch-list Data †Watch-List Threat although an emerging threat of mid-water trawling requires monitoring. -

A North Carolina Stranding of a White-Beaked Dolphin (Lagenorhynchus Albirostris), Family Delphinidae: a New Southerly Record Victoria G
Aquatic Mammals 2018, 44(1), 32-38 DOI 10.1578/AM.44.1.2018.32 A North Carolina Stranding of a White-Beaked Dolphin (Lagenorhynchus albirostris), Family Delphinidae: A New Southerly Record Victoria G. Thayer,1, 2 Craig A. Harms,3 Keith A. Rittmaster,4 David S. Rotstein,5 and John E. Hairr4 1North Carolina Division of Marine Fisheries, 3441 Arendell Street, Morehead City, NC 28557, USA E-mail: [email protected] 2North Carolina State University, Center for Marine Sciences and Technology, 303 College Circle, Morehead City, NC 28557, USA 3Department of Clinical Sciences, College of Veterinary Medicine and Center for Marine Sciences and Technology, North Carolina State University, 303 College Circle, Morehead City, NC 28557, USA 4North Carolina Maritime Museum, 315 Front Street, Beaufort, NC 28516, USA 5Marine Mammal Pathology Services, Olney, MD 20832, USA Abstract waters; and it is also seen in coastal waters (Kinze, 2002). The species is abundant and is listed as one An adult 273.7-kg, 266-cm male white-beaked of Least Concern by the International Union for dolphin (Lagenorhynchus albirostris) stranded Conservation of Nature (IUCN) as there have in Beaufort, North Carolina, on 16 April 2015 at been neither reported population declines nor 34.698125 N, -76.650476 W. Morphometrics, gross identified threats (Hammond et al., 2012). A pop- necropsy, and histopathologic evaluation were per- ulation minimum of several thousand is currently formed. Bilateral adrenal gland tumors (pheochro- estimated to exist in the northwestern Atlantic, mocytomas) were found and may have contributed and populations in the northeastern Atlantic may to stranding. This is the first recorded white-beaked be greater than 100,000 (Hammond et al., 2012). -

Figure2 Taxonomic Revision of the Dolphin Genus Lagenorhynchus
A) LeDuc et al. 1999, Figure 1 - cyt b (1,140 bp) B) May-Collado & Agnarsson 2006, Figure 2 - cyt b (578 or 1,140 bp) C) Agnarsson & May-Collado 2008, Figure 5 - cyt b (578 or 1,140 bp) 100/100/34 100 100/100 Phocoena spp. Phocoenidae Phocoenidae 65/92 100/100/23 85 Monodontidae 100/100 Feresa attenuata 100 Monodontidae Monodontidae 58/79/1 94/92 Peponocephala electra Orcaella brevirostris 98/99/6 100 Cephalorhynchus commersonii Orcinus orca Globicephala spp. 97/97/9 98 Cephalorhynchus eutropia 100/100 Globicephala spp. Grampus griseus 51/57 80 Cephalorhynchus hectori 55/69 Peponocephala electra Pseudorca crassidens Cephalorhynchus heavisidii 100/100 58/77 Feresa attenuata Orcinus orca 100/100 96 Lagenorhynchus australis Grampus griseus 98/89/9 Orcaella sp. Lagenorhynchus cruciger Pseudorca crassidens 100 99 Lagenorhynchus obliquidens 100/100/13 Lissodelphis borealis 100/100 Cephalorhynchus commersonii Lissodelphis peronii Lagenorhynchus obscurus 99/99 Cephalorhynchus eutropia 56/69/1 Lagenorhynchus obscurus 100 Lissodelphis borealis 73/78 Cephalorhynchus hectori Lagenorhynchus obliquidens Lissodelphis peronii 64/62 Cephalorhynchus heavisidii 100/100/9 100/99/8 Lagenorhynchus cruciger 100 27/X 100/100 Lagenorhynchus australis Delphinus sp. 95/96 Lagenorhynchus australis Lagenorhynchus cruciger 98/99/12 99 Cephalorhynchus heavisidii 59 95 Stenella clymene 100/100 100/100 Lagenorhynchus obliquidens 63/57/ Lagenorhynchus obscurus 2 Cephalorhynchus hectori 100 Stenella coeruleoalba Cephalorhynchus eutropia Stenella frontalis 100/100 Lissodelphis borealis 98/99/5 Cephalorhynchus commersonii 57 Tursiops truncatus Lissodelphis peronii Lagenodelphis hosei Lagenorhynchus albirostris 100/100 100 Sousa chinensis Delphinus sp. Lagenorhynchus acutus 77/77 * Stenella attenuata 99/99 Stenella clymene Steno bredanensis 69 93/89 * Stenella longirostris Stenella coeruleoalba 37/X 99/99 Sotalia fluviatilis 68 51 Sotalia fluviatilis 100 100/100 Stenella frontalis Steno bredanensis Sousa chinensis 43/88 Tursiops aduncus 76/78/2 Lagenorhynchus acutus Tursiops truncatus Stenella spp. -

Marine Mammal Taxonomy
Marine Mammal Taxonomy Kingdom: Animalia (Animals) Phylum: Chordata (Animals with notochords) Subphylum: Vertebrata (Vertebrates) Class: Mammalia (Mammals) Order: Cetacea (Cetaceans) Suborder: Mysticeti (Baleen Whales) Family: Balaenidae (Right Whales) Balaena mysticetus Bowhead whale Eubalaena australis Southern right whale Eubalaena glacialis North Atlantic right whale Eubalaena japonica North Pacific right whale Family: Neobalaenidae (Pygmy Right Whale) Caperea marginata Pygmy right whale Family: Eschrichtiidae (Grey Whale) Eschrichtius robustus Grey whale Family: Balaenopteridae (Rorquals) Balaenoptera acutorostrata Minke whale Balaenoptera bonaerensis Arctic Minke whale Balaenoptera borealis Sei whale Balaenoptera edeni Byrde’s whale Balaenoptera musculus Blue whale Balaenoptera physalus Fin whale Megaptera novaeangliae Humpback whale Order: Cetacea (Cetaceans) Suborder: Odontoceti (Toothed Whales) Family: Physeteridae (Sperm Whale) Physeter macrocephalus Sperm whale Family: Kogiidae (Pygmy and Dwarf Sperm Whales) Kogia breviceps Pygmy sperm whale Kogia sima Dwarf sperm whale DOLPHIN R ESEARCH C ENTER , 58901 Overseas Hwy, Grassy Key, FL 33050 (305) 289 -1121 www.dolphins.org Family: Platanistidae (South Asian River Dolphin) Platanista gangetica gangetica South Asian river dolphin (also known as Ganges and Indus river dolphins) Family: Iniidae (Amazon River Dolphin) Inia geoffrensis Amazon river dolphin (boto) Family: Lipotidae (Chinese River Dolphin) Lipotes vexillifer Chinese river dolphin (baiji) Family: Pontoporiidae (Franciscana) -

Molecular Systematics of South American Dolphins Sotalia: Sister
Available online at www.sciencedirect.com Molecular Phylogenetics and Evolution 46 (2008) 252–268 www.elsevier.com/locate/ympev Molecular systematics of South American dolphins Sotalia: Sister taxa determination and phylogenetic relationships, with insights into a multi-locus phylogeny of the Delphinidae Susana Caballero a,*, Jennifer Jackson a,g, Antonio A. Mignucci-Giannoni b, He´ctor Barrios-Garrido c, Sandra Beltra´n-Pedreros d, Marı´a G. Montiel-Villalobos e, Kelly M. Robertson f, C. Scott Baker a,g a Laboratory of Molecular Ecology and Evolution, School of Biological Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand b Red Cariben˜a de Varamientos, Caribbean Stranding Network, PO Box 361715, San Juan 00936-1715, Puerto Rico c Laboratorio de Ecologı´a General, Facultad Experimental de Ciencias. Universidad del Zulia, Av. Universidad con prolongacio´n Av. 5 de Julio. Sector Grano de Oro, Maracaibo, Venezuela d Laboratorio de Zoologia, Colecao Zoologica Paulo Burheim, Centro Universitario Luterano de Manaus, Manaus, Brazil e Laboratorio de Ecologı´a y Gene´tica de Poblaciones, Centro de Ecologı´a, Instituto Venezolano de Investigaciones Cientı´ficas (IVIC), San Antonio de los Altos, Carretera Panamericana km 11, Altos de Pipe, Estado Miranda, Venezuela f Tissue and DNA Archive, National Marine Fisheries Service, Southwest Fisheries Science Center, 8604 La Jolla Shores Drive, La Jolla, CA 92037-1508, USA g Marine Mammal Institute and Department of Fisheries and Wildlife, Hatfield Marine Science Center, Oregon State University, 2030 SE Marine Science Drive, Newport, OR 97365, USA Received 2 May 2007; revised 19 September 2007; accepted 17 October 2007 Available online 25 October 2007 Abstract The evolutionary relationships among members of the cetacean family Delphinidae, the dolphins, pilot whales and killer whales, are still not well understood. -

Marine Mammals of British Columbia Current Status, Distribution and Critical Habitats
Marine Mammals of British Columbia Current Status, Distribution and Critical Habitats John Ford and Linda Nichol Cetacean Research Program Pacific Biological Station Nanaimo, BC Outline • Brief (very) introduction to marine mammals of BC • Historical occurrence of whales in BC • Recent efforts to determine current status of cetacean species • Recent attempts to identify Critical Habitat for Threatened & Endangered species • Overview of pinnipeds in BC Marine Mammals of British Columbia - 25 Cetaceans, 5 Pinnipeds, 1 Mustelid Baleen Whales of British Columbia Family Balaenopteridae – Rorquals (5 spp) Blue Whale Balaenoptera musculus SARA Status = Endangered Fin Whale Balaenoptera physalus = Threatened = Spec. Concern Sei Whale Balaenoptera borealis Family Balaenidae – Right Whales (1 sp) Minke Whale Balaenoptera acutorostrata North Pacific Right Whale Eubalaena japonica Humpback Whale Megaptera novaeangliae Family Eschrichtiidae– Grey Whales (1 sp) Grey Whale Eschrichtius robustus Toothed Whales of British Columbia Family Physeteridae – Sperm Whales (3 spp) Sperm Whale Physeter macrocephalus Pygmy Sperm Whale Kogia breviceps Dwarf Sperm Whale Kogia sima Family Ziphiidae – Beaked Whales (4 spp) Hubbs’ Beaked Whale Mesoplodon carlhubbsii Stejneger’s Beaked Whale Mesoplodon stejnegeri Baird’s Beaked Whale Berardius bairdii Cuvier’s Beaked Whale Ziphius cavirostris Toothed Whales of British Columbia Family Delphinidae – Dolphins (9 spp) Pacific White-sided Dolphin Lagenorhynchus obliquidens Killer Whale Orcinus orca Striped Dolphin Stenella