Echolocation Click Parameters and Biosonar Behaviour of the Dwarf Sperm Whale (Kogia Sima) Chloe E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Is Harbor Porpoise (Phocoena Phocoena) Exhaled Breath Sampling Suitable for Hormonal Assessments?
animals Article Is Harbor Porpoise (Phocoena phocoena) Exhaled Breath Sampling Suitable for Hormonal Assessments? Anja Reckendorf 1,2 , Marion Schmicke 3 , Paulien Bunskoek 4, Kirstin Anderson Hansen 1,5, Mette Thybo 5, Christina Strube 2 and Ursula Siebert 1,* 1 Institute for Terrestrial and Aquatic Wildlife Research, University of Veterinary Medicine Hannover, Werftstrasse 6, 25761 Buesum, Germany; [email protected] (A.R.); [email protected] (K.A.H.) 2 Centre for Infection Medicine, Institute for Parasitology, University of Veterinary Medicine Hannover, Buenteweg 17, 30559 Hannover, Germany; [email protected] 3 Clinic for Cattle, Working Group Endocrinology, University of Veterinary Medicine Hannover, Bischofsholer Damm 15, 30173 Hannover, Germany; [email protected] 4 Dolfinarium, Zuiderzeeboulevard 22, 3841 WB Harderwijk, The Netherlands; paulien.bunskoek@dolfinarium.nl 5 Fjord & Bælt, Margrethes Pl. 1, 5300 Kerteminde, Denmark; [email protected] * Correspondence: [email protected]; Tel.: +49-511-856-8158 Simple Summary: The progress of animal welfare in wildlife conservation and research calls for more non-invasive sampling techniques. In cetaceans, exhaled breath condensate (blow)—a mixture of cells, mucus and fluids expelled through the force of a whale’s exhale—is a unique sampling matrix for hormones, bacteria and genetic material, among others. Especially the detection of steroid hormones, such as cortisol, is being investigated as stress indicators in several species. As the only Citation: Reckendorf, A.; Schmicke, native cetacean in Germany, harbor porpoises (Phocoena phocoena) are of special conservation concern M.; Bunskoek, P.; Anderson Hansen, and research interest. So far, strandings and live captures have been the only method to obtain K.; Thybo, M.; Strube, C.; Siebert, U. -

Single Particle Analysis of Particulate Pollutants in Yellowstone National Park During the Winter Snowmobile Season
SINGLE PARTICLE ANALYSIS OF PARTICULATE POLLUTANTS IN YELLOWSTONE NATIONAL PARK DURING THE WINTER SNOWMOBILE SEASON. Richard E. Peterson* and Bonnie J. Tyler** * Dept. of Chemistry and Biochemistry **Dept. of Chemical Engineering Montana State University, Bozeman, MT 59717, USA [email protected] Introduction: Particulate, or aerosol, pollution is a complex mixture of organic and inorganic compounds which includes a wide range of sizes and whose composition can vary widely depending on the time of year, geographical location, and both local and long range sources. Aerosol are important because of participation in atmospheric electricity, absorption and scattering of radiation (i.e. sunlight and thus affecting climate and visibility), their role as condensation nuclei for water vapor (and thus affecting precipitation chemistry and pH), and health effects. Particles greater than 2 micrometers in diameter (coarse) are generally formed by mechanical processes while smaller particles (fine) are formed by gas to particle conversion and accumulation/coagulation of these smallest aerosol. Because particles smaller than 2.5 micrometers (USEPA PM2.5) can become trapped deep in the lungs, it is of particular interest to identify toxic substances, such as heavy metals and polyaromatic hydrocarbons, that may be present in particles of this size range. Epidemiological studies have typically used particle size as the metric for identifying adverse health effects of particulate matter (PM), largely because data on PM size is available. Data on particle composition or other characteristics are less well known, if known at all in many cases. We are evaluating the potential for using Time of Flight Secondary Ion Mass Spectroscopy (TOF-SIMS) to study the composition of single particles from atmospheric aerosol. -

Understanding Harbour Porpoise (Phocoena Phocoena) and Fisheries Interactions in the North-West Iberian Peninsula
20th ASCOBANS Advisory Committee Meeting AC20/Doc.6.1.b (S) Warsaw, Poland, 27-29 August 2013 Dist. 11 July 2013 Agenda Item 6.1 Project Funding through ASCOBANS Progress of Supported Projects Document 6.1.b Project Report: Understanding harbour porpoise (Phocoena phocoena) and fisheries interactions in the north-west Iberian Peninsula Action Requested Take note Submitted by Secretariat / University of Aberdeen NOTE: DELEGATES ARE KINDLY REMINDED TO BRING THEIR OWN COPIES OF DOCUMENTS TO THE MEETING Final report to ASCOBANS (SSFA/ASCOBANS/2010/4) Understanding harbour porpoise (Phocoena phocoena) and fishery interactions in the north-west Iberian Peninsula Fiona L. Read1,2, M. Begoña Santos2,3, Ángel F. González1, Alfredo López4, Marisa Ferreira5, José Vingada5,6 and Graham J. Pierce2,3,6 1) Instituto de Investigaciones Marinas (C.S.I.C), Eduardo Cabello 6, 36208 Vigo, Spain 2) School of Biological Sciences (Zoology), University of Aberdeen, Tillydrone Avenue, Aberdeen, AB24 2TZ, Aberdeen, United Kingdom 3) Instituto Español de Oceanografía, Centro Oceanográfico de Vigo, PO Box 1552, 36200, Vigo, Spain 4) CEMMA, Apdo. 15, 36380, Gondomar, Spain 5) CBMA/SPVS, Departamento de Biologia, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal 6) CESAM, Departamento de Biologia, Universidade do Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal Coordinated by: In collaboration with: 1 Final report to ASCOBANS (SSFA/ASCOBANS/2010/4) Introduction The North West Iberian Peninsula (NWIP), as defined for the present project, consists of Galicia (north-west Spain), and north-central Portugal as far south as Peniche (Figure 1). Due to seasonal upwelling (Fraga, 1981), the NWIP sustains high productivity and high biodiversity, including almost 300 species of fish (Solørzano et al., 1988) and over 75 species of cephalopods (Guerra, 1992). -

Linking Mesopelagic Prey Abundance and Distribution to the Foraging
Deep–Sea Research Part II 140 (2017) 163–170 Contents lists available at ScienceDirect Deep–Sea Research II journal homepage: www.elsevier.com/locate/dsr2 Linking mesopelagic prey abundance and distribution to the foraging MARK behavior of a deep-diving predator, the northern elephant seal ⁎ Daisuke Saijoa,1, Yoko Mitanib,1, , Takuzo Abec,2, Hiroko Sasakid, Chandra Goetsche, Daniel P. Costae, Kazushi Miyashitab a Graduate School of Environmental Science, Hokkaido University, 20-5 Bentencho, Hakodate, Hokkaido 040-0051, Japan b Field Science Center for Northern Biosphere, Hokkaido University, 20-5 Bentencho, Hakodate, Hokkaido 040-0051, Japan c School of Fisheries Science, Hokkaido University, 3-1-1 Minato cho, Hakodate, Hokkaido 041-8611, Japan d Arctic Environment Research Center, National Institute of Polar Research, 10-3, Midori-cho, Tachikawa, Tokyo 190-8518, Japan e Department of Ecology & Evolutionary Biology, University of California, Santa Cruz, CA 95060, United States ARTICLE INFO ABSTRACT Keywords: The Transition Zone in the eastern North Pacific is important foraging habitat for many marine predators. Deep-scattering layer Further, the mesopelagic depths (200–1000 m) host an abundant prey resource known as the deep scattering Transition Zone layer that supports deep diving predators, such as northern elephant seals, beaked whales, and sperm whales. fi mesopelagic sh Female northern elephant seals (Mirounga angustirostris) undertake biannual foraging migrations to this myctophid region where they feed on mesopelagic fish and squid; however, in situ measurements of prey distribution and subsurface chlorophyll abundance, as well as the subsurface oceanographic features in the mesopelagic Transition Zone are limited. While concurrently tracking female elephant seals during their post-molt migration, we conducted a ship-based oceanographic and hydroacoustic survey and used mesopelagic mid-water trawls to sample the deep scattering layer. -

Taxonomic Status of the Genus Sotalia: Species Level Ranking for “Tucuxi” (Sotalia Fluviatilis) and “Costero” (Sotalia Guianensis) Dolphins
MARINE MAMMAL SCIENCE, **(*): ***–*** (*** 2007) C 2007 by the Society for Marine Mammalogy DOI: 10.1111/j.1748-7692.2007.00110.x TAXONOMIC STATUS OF THE GENUS SOTALIA: SPECIES LEVEL RANKING FOR “TUCUXI” (SOTALIA FLUVIATILIS) AND “COSTERO” (SOTALIA GUIANENSIS) DOLPHINS S. CABALLERO Laboratory of Molecular Ecology and Evolution, School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland, New Zealand and Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia F. TRUJILLO Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia J. A. VIANNA Sala L3–244, Departamento de Biologia Geral, ICB, Universidad Federal de Minas Gerais, Avenida Antonio Carlos, 6627 C. P. 486, 31270–010 Belo Horizonte, Brazil and Escuela de Medicina Veterinaria, Facultad de Ecologia y Recursos Naturales, Universidad Andres Bello Republica 252, Santigo, Chile H. BARRIOS-GARRIDO Laboratorio de Sistematica´ de Invertebrados Acuaticos´ (LASIA), Postgrado en Ciencias Biologicas,´ Facultad Experimental de Ciencias,Universidad del Zulia, Avenida Universidad con prolongacion´ Avenida 5 de Julio, Sector Grano de Oro, Maracaibo, Venezuela M. G. MONTIEL Laboratorio de Ecologıa´ y Genetica´ de Poblaciones, Centro de Ecologıa,´ Instituto Venezolano de Investigaciones Cientıficas´ (IVIC), San Antonio de los Altos, Carretera Panamericana km 11, Altos de Pipe, Estado Miranda, Venezuela S. BELTRAN´ -PEDREROS Laboratorio de Zoologia,´ Colec¸ao˜ Zoologica´ Paulo Burheim, Centro Universitario´ Luterano de Manaus, Manaus, Brazil 1 2 MARINE MAMMAL SCIENCE, VOL. **, NO. **, 2007 M. MARMONTEL Sociedade Civil Mamiraua,´ Rua Augusto Correa No.1 Campus do Guama,´ Setor Professional, Guama,´ C. P. 8600, 66075–110 Belem,´ Brazil M. C. SANTOS Projeto Atlantis/Instituto de Biologia da Conservac¸ao,˜ Laboratorio´ de Biologia da Conservac¸ao˜ de Cetaceos,´ Departamento de Zoologia, Universidade Estadual Paulista (UNESP), Campus Rio Claro, Sao˜ Paulo, Brazil M. -

Morphometrics of the Dolphin Genus Lagenorhynchus: Deciphering A
Morphometrics of the dolphin genus Lagenorhynchus: deciphering a contested phylogeny Allison Galezo1,2 and Nicole Vollmer1,3 1 Department of Vertebrate Zoology, Smithsonian Institution National Museum of Natural History 2 Department of Biology, Georgetown University 3 NOAA National Systematics Laboratory Background Results & Analysis Discussion • Our morphological data support the hypothesis that Figure 1. Phenogram from cluster analysis of dolphin skull Figure 2. Species symbols key with sample sizes. measurements. Calculated using Euclidian distances and the genus Lagenorhynchus is not monophyletic, evident Height l La. acutus (24) p C. commersonii (5) a b c Ward’s method. from the separation in the phenogram of La. albirostris Height p C. eutropia (2) Recent phylogenetic studies1-7 have indicated that the Distance l La. albirostris (10) and La. acutus from the other Lagenorhynchus species, 0 2 4 6 8 p genus Lagenorhynchus, currently containing the species 0 2 4 6 8 l La. australis (7) C. heavisidii (1) u Li. borealis (11) and the mix of genera in the lowermost clade (Figure 1). L. obliquidensa, L. acutusb , L. albirostrisc, L. obscurusd , L. l La. obliquidens (28) p C. hectori (2) n Unknown (1) • Our results show that La. obscurus and La. obliquidens e f cruciger , and L. australis , is not monophyletic. These C. commersonii l La. obscurus (15) are very similar morphologically, which supports the C. commersonii species were originally grouped together because of C.C. cocommemmersoniirsonii C. commeC. hersoniictori hypothesis that they are closely related: they have C. commeC. hersoniictori similarities in external morphology and coloration, but C. commeC. hersoniictori Figure 3. -
Global Patterns in Marine Mammal Distributions
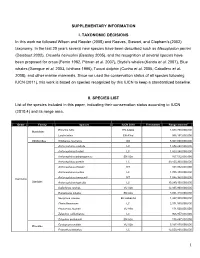
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

Censusing Non-Fish Nekton
WORKSHOP SYNOPSIS Censusing Non-Fish Nekton Carohln Levi, Gregory Stone and Jerry R. Schubel New England Aquarium ° Boston, Massachusetts USA his is a brief summary of a "Non-Fish SUMMARIES OF WORKING GROUPS Nekton" workshop held on 10-11 December 1997 at the New England Aquarium. The overall goals Cephalopods were: (1) to assess the feasibility of conducting a census New higher-level taxa are yet to be discovered, of life in the sea, (2) to identify the strategies and especially among coleoid cephalopods, which are components of such a census, (3) to assess whether a undergoing rapid evolutionary radiation. There are periodic census would generate scientifically worth- great gaps in natural history and ecosystem function- while results, and (4) to determine the level of interest ing, with even major commercial species largely of the scientific community in participating in the unknown. This is particularly, complex, since these design and conduct of a census of life in the sea. short-lived, rapidly growing animals move up through This workshop focused on "non-fish nekton," which trophic levels in a single season. were defined to include: marine mammals, marine reptiles, cephalopods and "other invertebrates." During . Early consolidation of existing cephalopod data is the course of the workshop, it was suggested that a needed, including the vast literatures in Japanese more appropriate phase for "other invertebrates" is and Russian. Access to and evaluation of historical invertebrate micronekton. Throughout the report we survey, catch, biological and video image data sets have used the latter terminology. and collections is needed. An Internet-based reposi- Birds were omitted only because of lack of time. -

Guidance for Using Continuous Monitors in Pm Monitoring
United States Office of Air Quality EPA-454/R-98-012 Environmental Protection Planning and Standards May 1998 Agency Research Triangle Park, NC 27711 Air GUIDANCE FOR USING CONTINUOUS MONITORS IN PM2.5 MONITORING NETWORKS GUIDANCE FOR USING CONTINUOUS MONITORS IN PM2.5 MONITORING NETWORKS May 29, 1998 PREPARED BY John G. Watson1 Judith C. Chow1 Hans Moosmüller1 Mark Green1 Neil Frank2 Marc Pitchford3 PREPARED FOR Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, NC 27711 1Desert Research Institute, University and Community College System of Nevada, PO Box 60220, Reno, NV 89506 2U.S. EPA/OAQPS, Research Triangle Park, NC, 27711 3National Oceanic and Atmospheric Administration, 755 E. Flamingo, Las Vegas, NV 89119 DISCLAIMER The development of this document has been funded by the U.S. Environmental Protection Agency, under cooperative agreement CX824291-01-1, and by the Desert Research Institute of the University and Community College System of Nevada. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. This draft has not been subject to the Agency’s peer and administrative review, and does not necessarily represent Agency policy or guidance. ii ABSTRACT This guidance provides a survey of alternatives for continuous in-situ measurements of suspended particles, their chemical components, and their gaseous precursors. Recent and anticipated advances in measurement technology provide reliable and practical instruments for particle quantification over averaging times ranging from minutes to hours. These devices provide instantaneous, telemetered results and can use limited manpower more efficiently than manual, filter-based methods. -

SHORT-FINNED PILOT WHALE (Globicephala Macrorhynchus): Western North Atlantic Stock
February 2019 SHORT-FINNED PILOT WHALE (Globicephala macrorhynchus): Western North Atlantic Stock STOCK DEFINITION AND GEOGRAPHIC RANGE There are two species of pilot whales in the western North Atlantic - the long-finned pilot whale, Globicephala melas melas, and the short-finned pilot whale, G. macrorhynchus. These species are difficult to differentiate at sea and cannot be reliably visually identified during either abundance surveys or observations of fishery mortality without high-quality photographs (Rone and Pace 2012); therefore, the ability to separately assess the two species in U.S. Atlantic waters is complex and requires additional information on seasonal spatial distribution. Pilot whales (Globicephala sp.) in the western North Atlantic occur primarily along the continental shelf break from Florida to the Nova Scotia Shelf (Mullin and Fulling 2003). Long-finned and short- finned pilot whales overlap spatially along the mid-Atlantic shelf break between Delaware and the southern flank of Georges Bank (Payne and Heinemann 1993; Rone and Pace 2012). Long-finned pilot whales have occasionally been observed stranded as far south as South Carolina, and short- finned pilot whales have occasionally been observed stranded as far north as Massachusetts (Pugliares et al. 2016). The exact latitudinal ranges of the two species remain uncertain. However, south of Cape Hatteras most pilot whale sightings are expected to be short- Figure 1. Distribution of long-finned (open symbols), short-finned finned pilot whales, while north of (black symbols), and possibly mixed (gray symbols; could be ~42°N most pilot whale sightings are either species) pilot whale sightings from NEFSC and SEFSC expected to be long-finned pilot whales shipboard and aerial surveys during the summers of 1998, 1999, (Figure 1; Garrison and Rosel 2017). -

Riverine and Marine Ecotypes of Sotalia Dolphins Are Different Species
Marine Biology (2005) 148: 449–457 DOI 10.1007/s00227-005-0078-2 RESEARCH ARTICLE H.A. Cunha Æ V.M.F. da Silva Æ J. Lailson-Brito Jr M.C.O. Santos Æ P.A.C. Flores Æ A.R. Martin A.F. Azevedo Æ A.B.L. Fragoso Æ R.C. Zanelatto A.M. Sole´-Cava Riverine and marine ecotypes of Sotalia dolphins are different species Received: 24 December 2004 / Accepted: 14 June 2005 / Published online: 6 September 2005 Ó Springer-Verlag 2005 Abstract The current taxonomic status of Sotalia species cific status of S. fluviatilis ecotypes and their population is uncertain. The genus once comprised five species, but structure along the Brazilian coast. Nested-clade (NCA), in the twentieth century they were grouped into two phylogenetic analyses and analysis of molecular variance (riverine Sotalia fluviatilis and marine Sotalia guianensis) of control region sequences showed that marine and that later were further lumped into a single species riverine ecotypes form very divergent monophyletic (S. fluviatilis), with marine and riverine ecotypes. This groups (2.5% sequence divergence; 75% of total molec- uncertainty hampers the assessment of potential impacts ular variance found between them), which have been on populations and the design of effective conservation evolving independently since an old allopatric fragmen- measures. We used mitochondrial DNA control region tation event. This result is also corroborated by cyto- and cytochrome b sequence data to investigate the spe- chrome b sequence data, for which marine and riverine specimens are fixed for haplotypes that differ by 28 (out Communicated by J. P. -

Kogia Species Guild
Supplemental Volume: Species of Conservation Concern SC SWAP 2015 Sperm Whales Guild Dwarf sperm whale (Kogia sima) Pygmy sperm whale (Kogia breviceps) Contributor (2005): Wayne McFee (NOAA) Reviewed and Edited (2012): Wayne McFee (NOAA) DESCRIPTION Taxonomy and Basic Description The pygmy sperm whale was first described by de Blainville in 1838. The dwarf sperm whale was first described by Owen in 1866. Both were considered a Illustration by Pieter A. Folkens single species until 1966. These are the only two species in the family Kogiidae. The species name for the dwarf sperm whale was changed in 1998 from ‘simus’ to ‘sima.’ Neither the pygmy nor dwarf sperm whale are kin to the true sperm whale (Physeter macrocephalus). At sea, these two species are virtually indistinguishable. Both species are black dorsally with a white underside. They possess a shark-like head with a narrow under-slung lower jaw and a light colored “false gill” that runs between the eye and the flipper. Small flippers are positioned far forward on the body. Pygmy sperm whales generally have between 12 and 16 (occasionally 10 to 11) pairs of needle- like teeth in the lower jaw. They can attain lengths up to 3.5 m (11.5 ft.) and weigh upwards of 410 kg (904 lbs.). A diagnostic character of this species is the low, falcate dorsal fin (less than 5% of the body length) positioned behind the midpoint on the back. Dwarf sperm whales generally have 8 to 11 (rarely up to 13) pairs of teeth in the lower jaw and can have up to 3 pairs of teeth in the upper jaw.