Spatial Distribution of Rorqual Whales in the Strait of Jacques Cartier, Gulf of St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Is Harbor Porpoise (Phocoena Phocoena) Exhaled Breath Sampling Suitable for Hormonal Assessments?
animals Article Is Harbor Porpoise (Phocoena phocoena) Exhaled Breath Sampling Suitable for Hormonal Assessments? Anja Reckendorf 1,2 , Marion Schmicke 3 , Paulien Bunskoek 4, Kirstin Anderson Hansen 1,5, Mette Thybo 5, Christina Strube 2 and Ursula Siebert 1,* 1 Institute for Terrestrial and Aquatic Wildlife Research, University of Veterinary Medicine Hannover, Werftstrasse 6, 25761 Buesum, Germany; [email protected] (A.R.); [email protected] (K.A.H.) 2 Centre for Infection Medicine, Institute for Parasitology, University of Veterinary Medicine Hannover, Buenteweg 17, 30559 Hannover, Germany; [email protected] 3 Clinic for Cattle, Working Group Endocrinology, University of Veterinary Medicine Hannover, Bischofsholer Damm 15, 30173 Hannover, Germany; [email protected] 4 Dolfinarium, Zuiderzeeboulevard 22, 3841 WB Harderwijk, The Netherlands; paulien.bunskoek@dolfinarium.nl 5 Fjord & Bælt, Margrethes Pl. 1, 5300 Kerteminde, Denmark; [email protected] * Correspondence: [email protected]; Tel.: +49-511-856-8158 Simple Summary: The progress of animal welfare in wildlife conservation and research calls for more non-invasive sampling techniques. In cetaceans, exhaled breath condensate (blow)—a mixture of cells, mucus and fluids expelled through the force of a whale’s exhale—is a unique sampling matrix for hormones, bacteria and genetic material, among others. Especially the detection of steroid hormones, such as cortisol, is being investigated as stress indicators in several species. As the only Citation: Reckendorf, A.; Schmicke, native cetacean in Germany, harbor porpoises (Phocoena phocoena) are of special conservation concern M.; Bunskoek, P.; Anderson Hansen, and research interest. So far, strandings and live captures have been the only method to obtain K.; Thybo, M.; Strube, C.; Siebert, U. -

WWII Veterans Last Name L
<%@LANGUAGE="JAVASCRIPT" CODEPAGE="65001"%> WWII Veterans last name L Labbe, Lawrence A. 9 Lea St. Label, Raoul 33 Hampton St. Labell, Morris 10 Concord St. LaBelle, Leo Desire 78 Exchange St. LaBelle, Paul A. 27 Farley St. Laberge, Fernand A. 444 Haverhill St. LaBonte, Andrew Shea 1085 Essex St. Labonte, Jean P. 439 South Broadway Labonte, John P. 439 South Broadway Labonte, Louis J. 5 Atkinson St. Labonte, Paul 439 South Broadway Labranche, Alfred 507 Essex St. Labranche, Armand C. 16 Bennett St. LaBranche, Claude G. 12 Tremont St. Labrecque, George A. Labrecque, Gerard J. Labrecque, John W. 13 Wyman St. Labrecque, Rene A. 108 Margin St. Labrie, Lionel 6 Cedar St. Lacaillade, Francis M. Lacaillade, Maurice C. LaCarte, John L. 28 Congress St. LaCarubba, John S. 34 Wilmot St. LaCarubba, Mario J. 34 Wilmot St. Lacasse, Albert J. 329 Broadway Lacasse, Andrew L. Lacasse, Arthur P. 329 Broadway Lacasse, Edward R. 329 Broadway Lacasse, Hubert L. 88 Columbus Ave. Lacasse, Joseph Lacasse, Wilfred V. 152 Spruce St. Lacey, Alphonse M. Lacey, Julian J. 57 Juniper St. Lacey, Richard L. 49 Avon St. LaChance, Albert Elizard 35 Tremont St. LaChance, Arthur R. 35 Irene St. LaChance, Julian 57 Groton St. LaChance, Ronald J. Lachance, Victor T. LaChapelle, Wilbrod A. 154 Margin St. Lacharite, Edward A. 34 Bellevue St. Lacharite, Edward A. 34 Bellevue St. 1 of 14 LaCharite, Leonard LaCharite, Robert F. 99 Arlington St. Lacharite, Wilfred E. Lacolla, Vincent 35 Sargent St. Lacourse, George J. Lacouter, Francis Roy Lacroix, Adelard Lacroix, Ernest Wilfred LaCroix, Henry E. Oxford St. Lacroix, Lionel 327 South Broadway LaCroix, Louis Lacroix, Maurice J. -

Understanding Harbour Porpoise (Phocoena Phocoena) and Fisheries Interactions in the North-West Iberian Peninsula
20th ASCOBANS Advisory Committee Meeting AC20/Doc.6.1.b (S) Warsaw, Poland, 27-29 August 2013 Dist. 11 July 2013 Agenda Item 6.1 Project Funding through ASCOBANS Progress of Supported Projects Document 6.1.b Project Report: Understanding harbour porpoise (Phocoena phocoena) and fisheries interactions in the north-west Iberian Peninsula Action Requested Take note Submitted by Secretariat / University of Aberdeen NOTE: DELEGATES ARE KINDLY REMINDED TO BRING THEIR OWN COPIES OF DOCUMENTS TO THE MEETING Final report to ASCOBANS (SSFA/ASCOBANS/2010/4) Understanding harbour porpoise (Phocoena phocoena) and fishery interactions in the north-west Iberian Peninsula Fiona L. Read1,2, M. Begoña Santos2,3, Ángel F. González1, Alfredo López4, Marisa Ferreira5, José Vingada5,6 and Graham J. Pierce2,3,6 1) Instituto de Investigaciones Marinas (C.S.I.C), Eduardo Cabello 6, 36208 Vigo, Spain 2) School of Biological Sciences (Zoology), University of Aberdeen, Tillydrone Avenue, Aberdeen, AB24 2TZ, Aberdeen, United Kingdom 3) Instituto Español de Oceanografía, Centro Oceanográfico de Vigo, PO Box 1552, 36200, Vigo, Spain 4) CEMMA, Apdo. 15, 36380, Gondomar, Spain 5) CBMA/SPVS, Departamento de Biologia, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal 6) CESAM, Departamento de Biologia, Universidade do Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal Coordinated by: In collaboration with: 1 Final report to ASCOBANS (SSFA/ASCOBANS/2010/4) Introduction The North West Iberian Peninsula (NWIP), as defined for the present project, consists of Galicia (north-west Spain), and north-central Portugal as far south as Peniche (Figure 1). Due to seasonal upwelling (Fraga, 1981), the NWIP sustains high productivity and high biodiversity, including almost 300 species of fish (Solørzano et al., 1988) and over 75 species of cephalopods (Guerra, 1992). -

Order CETACEA Suborder MYSTICETI BALAENIDAE Eubalaena Glacialis (Müller, 1776) EUG En - Northern Right Whale; Fr - Baleine De Biscaye; Sp - Ballena Franca
click for previous page Cetacea 2041 Order CETACEA Suborder MYSTICETI BALAENIDAE Eubalaena glacialis (Müller, 1776) EUG En - Northern right whale; Fr - Baleine de Biscaye; Sp - Ballena franca. Adults common to 17 m, maximum to 18 m long.Body rotund with head to 1/3 of total length;no pleats in throat; dorsal fin absent. Mostly black or dark brown, may have white splotches on chin and belly.Commonly travel in groups of less than 12 in shallow water regions. IUCN Status: Endangered. BALAENOPTERIDAE Balaenoptera acutorostrata Lacepède, 1804 MIW En - Minke whale; Fr - Petit rorqual; Sp - Rorcual enano. Adult males maximum to slightly over 9 m long, females to 10.7 m.Head extremely pointed with prominent me- dian ridge. Body dark grey to black dorsally and white ventrally with streaks and lobes of intermediate shades along sides.Commonly travel singly or in groups of 2 or 3 in coastal and shore areas;may be found in groups of several hundred on feeding grounds. IUCN Status: Lower risk, near threatened. Balaenoptera borealis Lesson, 1828 SIW En - Sei whale; Fr - Rorqual de Rudolphi; Sp - Rorcual del norte. Adults to 18 m long. Typical rorqual body shape; dorsal fin tall and strongly curved, rises at a steep angle from back.Colour of body is mostly dark grey or blue-grey with a whitish area on belly and ventral pleats.Commonly travel in groups of 2 to 5 in open ocean waters. IUCN Status: Endangered. 2042 Marine Mammals Balaenoptera edeni Anderson, 1878 BRW En - Bryde’s whale; Fr - Rorqual de Bryde; Sp - Rorcual tropical. -

Cetaceans: Whales and Dolphins
CETACEANS: WHALES AND DOLPHINS By Anna Plattner Objective Students will explore the natural history of whales and dolphins around the world. Content will be focused on how whales and dolphins are adapted to the marine environment, the differences between toothed and baleen whales, and how whales and dolphins communicate and find food. Characteristics of specific species of whales will be presented throughout the guide. What is a cetacean? A cetacean is any marine mammal in the order Cetaceae. These animals live their entire lives in water and include whales, dolphins, and porpoises. There are 81 known species of whales, dolphins, and porpoises. The two suborders of cetaceans are mysticetes (baleen whales) and odontocetes (toothed whales). Cetaceans are mammals, thus they are warm blooded, give live birth, have hair when they are born (most lose their hair soon after), and nurse their young. How are cetaceans adapted to the marine environment? Cetaceans have developed many traits that allow them to thrive in the marine environment. They have streamlined bodies that glide easily through the water and help them conserve energy while they swim. Cetaceans breathe through a blowhole, located on the top of their head. This allows them to float at the surface of the water and easily exhale and inhale. Cetaceans also have a thick layer of fat tissue called blubber that insulates their internals organs and muscles. The limbs of cetaceans have also been modified for swimming. A cetacean has a powerful tailfin called a fluke and forelimbs called flippers that help them steer through the water. Most cetaceans also have a dorsal fin that helps them stabilize while swimming. -

Fall12 Rare Southern California Sperm Whale Sighting
Rare Southern California Sperm Whale Sighting Dolphin/Whale Interaction Is Unique IN MAY 2011, a rare occurrence The sperm whale sighting off San of 67 minutes as the whales traveled took place off the Southern California Diego was exciting not only because slowly east and out over the edge of coast. For the first time since U.S. of its rarity, but because there were the underwater ridge. The adult Navy-funded aerial surveys began in also two species of dolphins, sperm whales undertook two long the area in 2008, a group of 20 sperm northern right whale dolphins and dives lasting about 20 minutes each; whales, including four calves, was Risso’s dolphins, interacting with the the calves surfaced earlier, usually in seen—approximately 24 nautical sperm whales in a remarkable the company of one adult whale. miles west of San Diego. manner. To the knowledge of the During these dives, the dolphins researchers who conducted this aerial remained at the surface and Operating under a National Marine survey, this type of inter-species asso- appeared to wait for the sperm Fisheries Service (NMFS) permit, the ciation has not been previously whales to re-surface. U.S. Navy has been conducting aerial reported. Video and photographs surveys of marine mammal and sea Several minutes after the sperm were taken of the group over a period turtle behavior in the near shore and whales were first seen, the Risso’s offshore waters within the Southern California Range Complex (SOCAL) since 2008. During a routine survey the morning of 14 May 2011, the sperm whales were sighted on the edge of an offshore bank near a steep drop-off. -

Taxonomic Status of the Genus Sotalia: Species Level Ranking for “Tucuxi” (Sotalia Fluviatilis) and “Costero” (Sotalia Guianensis) Dolphins
MARINE MAMMAL SCIENCE, **(*): ***–*** (*** 2007) C 2007 by the Society for Marine Mammalogy DOI: 10.1111/j.1748-7692.2007.00110.x TAXONOMIC STATUS OF THE GENUS SOTALIA: SPECIES LEVEL RANKING FOR “TUCUXI” (SOTALIA FLUVIATILIS) AND “COSTERO” (SOTALIA GUIANENSIS) DOLPHINS S. CABALLERO Laboratory of Molecular Ecology and Evolution, School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland, New Zealand and Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia F. TRUJILLO Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia J. A. VIANNA Sala L3–244, Departamento de Biologia Geral, ICB, Universidad Federal de Minas Gerais, Avenida Antonio Carlos, 6627 C. P. 486, 31270–010 Belo Horizonte, Brazil and Escuela de Medicina Veterinaria, Facultad de Ecologia y Recursos Naturales, Universidad Andres Bello Republica 252, Santigo, Chile H. BARRIOS-GARRIDO Laboratorio de Sistematica´ de Invertebrados Acuaticos´ (LASIA), Postgrado en Ciencias Biologicas,´ Facultad Experimental de Ciencias,Universidad del Zulia, Avenida Universidad con prolongacion´ Avenida 5 de Julio, Sector Grano de Oro, Maracaibo, Venezuela M. G. MONTIEL Laboratorio de Ecologıa´ y Genetica´ de Poblaciones, Centro de Ecologıa,´ Instituto Venezolano de Investigaciones Cientıficas´ (IVIC), San Antonio de los Altos, Carretera Panamericana km 11, Altos de Pipe, Estado Miranda, Venezuela S. BELTRAN´ -PEDREROS Laboratorio de Zoologia,´ Colec¸ao˜ Zoologica´ Paulo Burheim, Centro Universitario´ Luterano de Manaus, Manaus, Brazil 1 2 MARINE MAMMAL SCIENCE, VOL. **, NO. **, 2007 M. MARMONTEL Sociedade Civil Mamiraua,´ Rua Augusto Correa No.1 Campus do Guama,´ Setor Professional, Guama,´ C. P. 8600, 66075–110 Belem,´ Brazil M. C. SANTOS Projeto Atlantis/Instituto de Biologia da Conservac¸ao,˜ Laboratorio´ de Biologia da Conservac¸ao˜ de Cetaceos,´ Departamento de Zoologia, Universidade Estadual Paulista (UNESP), Campus Rio Claro, Sao˜ Paulo, Brazil M. -

Morphometrics of the Dolphin Genus Lagenorhynchus: Deciphering A
Morphometrics of the dolphin genus Lagenorhynchus: deciphering a contested phylogeny Allison Galezo1,2 and Nicole Vollmer1,3 1 Department of Vertebrate Zoology, Smithsonian Institution National Museum of Natural History 2 Department of Biology, Georgetown University 3 NOAA National Systematics Laboratory Background Results & Analysis Discussion • Our morphological data support the hypothesis that Figure 1. Phenogram from cluster analysis of dolphin skull Figure 2. Species symbols key with sample sizes. measurements. Calculated using Euclidian distances and the genus Lagenorhynchus is not monophyletic, evident Height l La. acutus (24) p C. commersonii (5) a b c Ward’s method. from the separation in the phenogram of La. albirostris Height p C. eutropia (2) Recent phylogenetic studies1-7 have indicated that the Distance l La. albirostris (10) and La. acutus from the other Lagenorhynchus species, 0 2 4 6 8 p genus Lagenorhynchus, currently containing the species 0 2 4 6 8 l La. australis (7) C. heavisidii (1) u Li. borealis (11) and the mix of genera in the lowermost clade (Figure 1). L. obliquidensa, L. acutusb , L. albirostrisc, L. obscurusd , L. l La. obliquidens (28) p C. hectori (2) n Unknown (1) • Our results show that La. obscurus and La. obliquidens e f cruciger , and L. australis , is not monophyletic. These C. commersonii l La. obscurus (15) are very similar morphologically, which supports the C. commersonii species were originally grouped together because of C.C. cocommemmersoniirsonii C. commeC. hersoniictori hypothesis that they are closely related: they have C. commeC. hersoniictori similarities in external morphology and coloration, but C. commeC. hersoniictori Figure 3. -
Global Patterns in Marine Mammal Distributions
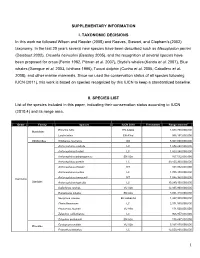
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

The Municipal Reform Movement in Montreal, 1886-1914. Miche A
The Municipal Reform Movement in Montreal, 1886-1914. Miche LIBRARIES » A thesis submitted in conformity with the requirement for the degree of Master of Arts in the University of Ottawa. 1972. Michel Gauvin, Ottawa 1972. UMI Number: EC55653 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. UMI® UMI Microform EC55653 Copyright 2011 by ProQuest LLC All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Abstract This thesis will prove that the Montreal reform movement arose out of a desire of the richer wards to control municipal politics. The poorer wards received most of the public works because they were organized into a political machine. Calling for an end to dishonesty and extravagance, the reform movement took on a party form. However after the leader of the machine retired from municipal politics, the movement lost its momentum. The reform movement eventually became identified as an anti-trust movement. The gas, electricity, and tramway utilities had usually obtained any contracts they wished, but the reformers put an end to this. -

SHORT-FINNED PILOT WHALE (Globicephala Macrorhynchus): Western North Atlantic Stock
February 2019 SHORT-FINNED PILOT WHALE (Globicephala macrorhynchus): Western North Atlantic Stock STOCK DEFINITION AND GEOGRAPHIC RANGE There are two species of pilot whales in the western North Atlantic - the long-finned pilot whale, Globicephala melas melas, and the short-finned pilot whale, G. macrorhynchus. These species are difficult to differentiate at sea and cannot be reliably visually identified during either abundance surveys or observations of fishery mortality without high-quality photographs (Rone and Pace 2012); therefore, the ability to separately assess the two species in U.S. Atlantic waters is complex and requires additional information on seasonal spatial distribution. Pilot whales (Globicephala sp.) in the western North Atlantic occur primarily along the continental shelf break from Florida to the Nova Scotia Shelf (Mullin and Fulling 2003). Long-finned and short- finned pilot whales overlap spatially along the mid-Atlantic shelf break between Delaware and the southern flank of Georges Bank (Payne and Heinemann 1993; Rone and Pace 2012). Long-finned pilot whales have occasionally been observed stranded as far south as South Carolina, and short- finned pilot whales have occasionally been observed stranded as far north as Massachusetts (Pugliares et al. 2016). The exact latitudinal ranges of the two species remain uncertain. However, south of Cape Hatteras most pilot whale sightings are expected to be short- Figure 1. Distribution of long-finned (open symbols), short-finned finned pilot whales, while north of (black symbols), and possibly mixed (gray symbols; could be ~42°N most pilot whale sightings are either species) pilot whale sightings from NEFSC and SEFSC expected to be long-finned pilot whales shipboard and aerial surveys during the summers of 1998, 1999, (Figure 1; Garrison and Rosel 2017). -

Whale Identification Posters
Whale dorsal fins Humpback whale Sperm whale Photo: Nadine Bott Photo: Whale Watch Kaikoura Blue whale Southern right whale (no dorsal) Photo: Nadine Bott Photo: Nadine Bott False killer whale Pilot whale Photo: Jochen Zaeschmar Photo: Jochen Zaeschmar • Report all marine mammal sightings to DOC via the online sighting form (www.doc.govt.nz/marinemammalsightings) • Email photos of marine mammal sightings to [email protected] Thank you for your help! 24hr emergency hotline Orca Photo: Ailie Suzuki Keep your distance – do not approach closer than 50 m to whales Whale tail flukes Humpback whale Sperm whale Photo: Nadine Bott Photo: Nadine Bott Blue whale Southern right whale Photo: Gregory Smith (CC BY-SA 2.0) Photo: Tui De Roy False killer whale Pilot whale Photo: Jochen Zaeschmar Photo: Jochen Zaeschmar • Report all marine mammal sightings to DOC via the online sighting form (www.doc.govt.nz/marinemammalsightings) • Email photos of marine mammal sightings to [email protected] Thank you for your help! 24hr emergency hotline Orca Photo: Nadine Bott Keep your distance – do not approach closer than 50 m to whales Whale surface behaviour Humpback whale Sperm whale Photo: Nadine Bott Photo: Whale Watch Kaikoura Blue whale Southern right whale Photo: Jochen Zaeschmar Photo: Don Goodhue False killer whale Pilot whale Photo: Jochen Zaeschmar Photo: Jochen Zaeschmar • Report all marine mammal sightings to DOC via the online sighting form (www.doc.govt.nz/marinemammalsightings) • Email photos of marine mammal sightings to [email protected]