Sotalia Fluviatilis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Marine Mammal Species and Subspecies Written by The
List of Marine Mammal Species and Subspecies Written by the Committee on Taxonomy The Ad-Hoc Committee on Taxonomy , chaired by Bill Perrin, has produced the first official SMM list of marine mammal species and subspecies. Consensus on some issues was not possible; this is reflected in the footnotes. This list will be revisited and possibly revised every few months reflecting the continuing flux in marine mammal taxonomy. This list can be cited as follows: “Committee on Taxonomy. 2009. List of marine mammal species and subspecies. Society for Marine Mammalogy, www.marinemammalscience.org, consulted on [date].” This list includes living and recently extinct species and subspecies. It is meant to reflect prevailing usage and recent revisions published in the peer-reviewed literature. Author(s) and year of description of the species follow the Latin species name; when these are enclosed in parentheses, the species was originally described in a different genus. Classification and scientific names follow Rice (1998), with adjustments reflecting more recent literature. Common names are arbitrary and change with time and place; one or two currently frequently used in English and/or a range language are given here. Additional English common names and common names in French, Spanish, Russian and other languages are available at www.marinespecies.org/cetacea/ . The cetaceans genetically and morphologically fall firmly within the artiodactyl clade (Geisler and Uhen, 2005), and therefore we include them in the order Cetartiodactyla, with Cetacea, Mysticeti and Odontoceti as unranked taxa (recognizing that the classification within Cetartiodactyla remains partially unresolved -- e.g., see Spaulding et al ., 2009) 1. -

THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A
s l a m m a y t T i M S N v I i A e G t A n i p E S r a A C a C E H n T M i THE CASE AGAINST Marine Mammals in Captivity The Humane Society of the United State s/ World Society for the Protection of Animals 2009 1 1 1 2 0 A M , n o t s o g B r o . 1 a 0 s 2 u - e a t i p s u S w , t e e r t S h t u o S 9 8 THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A. Rose, E.C.M. Parsons, and Richard Farinato, 4th edition Editors: Naomi A. Rose and Debra Firmani, 4th edition ©2009 The Humane Society of the United States and the World Society for the Protection of Animals. All rights reserved. ©2008 The HSUS. All rights reserved. Printed on recycled paper, acid free and elemental chlorine free, with soy-based ink. Cover: ©iStockphoto.com/Ying Ying Wong Overview n the debate over marine mammals in captivity, the of the natural environment. The truth is that marine mammals have evolved physically and behaviorally to survive these rigors. public display industry maintains that marine mammal For example, nearly every kind of marine mammal, from sea lion Iexhibits serve a valuable conservation function, people to dolphin, travels large distances daily in a search for food. In learn important information from seeing live animals, and captivity, natural feeding and foraging patterns are completely lost. -

Taxonomic Status of the Genus Sotalia: Species Level Ranking for “Tucuxi” (Sotalia Fluviatilis) and “Costero” (Sotalia Guianensis) Dolphins
MARINE MAMMAL SCIENCE, **(*): ***–*** (*** 2007) C 2007 by the Society for Marine Mammalogy DOI: 10.1111/j.1748-7692.2007.00110.x TAXONOMIC STATUS OF THE GENUS SOTALIA: SPECIES LEVEL RANKING FOR “TUCUXI” (SOTALIA FLUVIATILIS) AND “COSTERO” (SOTALIA GUIANENSIS) DOLPHINS S. CABALLERO Laboratory of Molecular Ecology and Evolution, School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland, New Zealand and Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia F. TRUJILLO Fundacion´ Omacha, Diagonal 86A #30–38, Bogota,´ Colombia J. A. VIANNA Sala L3–244, Departamento de Biologia Geral, ICB, Universidad Federal de Minas Gerais, Avenida Antonio Carlos, 6627 C. P. 486, 31270–010 Belo Horizonte, Brazil and Escuela de Medicina Veterinaria, Facultad de Ecologia y Recursos Naturales, Universidad Andres Bello Republica 252, Santigo, Chile H. BARRIOS-GARRIDO Laboratorio de Sistematica´ de Invertebrados Acuaticos´ (LASIA), Postgrado en Ciencias Biologicas,´ Facultad Experimental de Ciencias,Universidad del Zulia, Avenida Universidad con prolongacion´ Avenida 5 de Julio, Sector Grano de Oro, Maracaibo, Venezuela M. G. MONTIEL Laboratorio de Ecologıa´ y Genetica´ de Poblaciones, Centro de Ecologıa,´ Instituto Venezolano de Investigaciones Cientıficas´ (IVIC), San Antonio de los Altos, Carretera Panamericana km 11, Altos de Pipe, Estado Miranda, Venezuela S. BELTRAN´ -PEDREROS Laboratorio de Zoologia,´ Colec¸ao˜ Zoologica´ Paulo Burheim, Centro Universitario´ Luterano de Manaus, Manaus, Brazil 1 2 MARINE MAMMAL SCIENCE, VOL. **, NO. **, 2007 M. MARMONTEL Sociedade Civil Mamiraua,´ Rua Augusto Correa No.1 Campus do Guama,´ Setor Professional, Guama,´ C. P. 8600, 66075–110 Belem,´ Brazil M. C. SANTOS Projeto Atlantis/Instituto de Biologia da Conservac¸ao,˜ Laboratorio´ de Biologia da Conservac¸ao˜ de Cetaceos,´ Departamento de Zoologia, Universidade Estadual Paulista (UNESP), Campus Rio Claro, Sao˜ Paulo, Brazil M. -

Morphometrics of the Dolphin Genus Lagenorhynchus: Deciphering A
Morphometrics of the dolphin genus Lagenorhynchus: deciphering a contested phylogeny Allison Galezo1,2 and Nicole Vollmer1,3 1 Department of Vertebrate Zoology, Smithsonian Institution National Museum of Natural History 2 Department of Biology, Georgetown University 3 NOAA National Systematics Laboratory Background Results & Analysis Discussion • Our morphological data support the hypothesis that Figure 1. Phenogram from cluster analysis of dolphin skull Figure 2. Species symbols key with sample sizes. measurements. Calculated using Euclidian distances and the genus Lagenorhynchus is not monophyletic, evident Height l La. acutus (24) p C. commersonii (5) a b c Ward’s method. from the separation in the phenogram of La. albirostris Height p C. eutropia (2) Recent phylogenetic studies1-7 have indicated that the Distance l La. albirostris (10) and La. acutus from the other Lagenorhynchus species, 0 2 4 6 8 p genus Lagenorhynchus, currently containing the species 0 2 4 6 8 l La. australis (7) C. heavisidii (1) u Li. borealis (11) and the mix of genera in the lowermost clade (Figure 1). L. obliquidensa, L. acutusb , L. albirostrisc, L. obscurusd , L. l La. obliquidens (28) p C. hectori (2) n Unknown (1) • Our results show that La. obscurus and La. obliquidens e f cruciger , and L. australis , is not monophyletic. These C. commersonii l La. obscurus (15) are very similar morphologically, which supports the C. commersonii species were originally grouped together because of C.C. cocommemmersoniirsonii C. commeC. hersoniictori hypothesis that they are closely related: they have C. commeC. hersoniictori similarities in external morphology and coloration, but C. commeC. hersoniictori Figure 3. -
Global Patterns in Marine Mammal Distributions
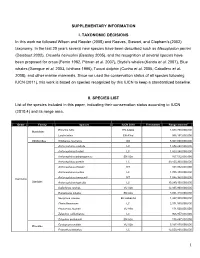
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

SHORT-FINNED PILOT WHALE (Globicephala Macrorhynchus): Western North Atlantic Stock
February 2019 SHORT-FINNED PILOT WHALE (Globicephala macrorhynchus): Western North Atlantic Stock STOCK DEFINITION AND GEOGRAPHIC RANGE There are two species of pilot whales in the western North Atlantic - the long-finned pilot whale, Globicephala melas melas, and the short-finned pilot whale, G. macrorhynchus. These species are difficult to differentiate at sea and cannot be reliably visually identified during either abundance surveys or observations of fishery mortality without high-quality photographs (Rone and Pace 2012); therefore, the ability to separately assess the two species in U.S. Atlantic waters is complex and requires additional information on seasonal spatial distribution. Pilot whales (Globicephala sp.) in the western North Atlantic occur primarily along the continental shelf break from Florida to the Nova Scotia Shelf (Mullin and Fulling 2003). Long-finned and short- finned pilot whales overlap spatially along the mid-Atlantic shelf break between Delaware and the southern flank of Georges Bank (Payne and Heinemann 1993; Rone and Pace 2012). Long-finned pilot whales have occasionally been observed stranded as far south as South Carolina, and short- finned pilot whales have occasionally been observed stranded as far north as Massachusetts (Pugliares et al. 2016). The exact latitudinal ranges of the two species remain uncertain. However, south of Cape Hatteras most pilot whale sightings are expected to be short- Figure 1. Distribution of long-finned (open symbols), short-finned finned pilot whales, while north of (black symbols), and possibly mixed (gray symbols; could be ~42°N most pilot whale sightings are either species) pilot whale sightings from NEFSC and SEFSC expected to be long-finned pilot whales shipboard and aerial surveys during the summers of 1998, 1999, (Figure 1; Garrison and Rosel 2017). -

Riverine and Marine Ecotypes of Sotalia Dolphins Are Different Species
Marine Biology (2005) 148: 449–457 DOI 10.1007/s00227-005-0078-2 RESEARCH ARTICLE H.A. Cunha Æ V.M.F. da Silva Æ J. Lailson-Brito Jr M.C.O. Santos Æ P.A.C. Flores Æ A.R. Martin A.F. Azevedo Æ A.B.L. Fragoso Æ R.C. Zanelatto A.M. Sole´-Cava Riverine and marine ecotypes of Sotalia dolphins are different species Received: 24 December 2004 / Accepted: 14 June 2005 / Published online: 6 September 2005 Ó Springer-Verlag 2005 Abstract The current taxonomic status of Sotalia species cific status of S. fluviatilis ecotypes and their population is uncertain. The genus once comprised five species, but structure along the Brazilian coast. Nested-clade (NCA), in the twentieth century they were grouped into two phylogenetic analyses and analysis of molecular variance (riverine Sotalia fluviatilis and marine Sotalia guianensis) of control region sequences showed that marine and that later were further lumped into a single species riverine ecotypes form very divergent monophyletic (S. fluviatilis), with marine and riverine ecotypes. This groups (2.5% sequence divergence; 75% of total molec- uncertainty hampers the assessment of potential impacts ular variance found between them), which have been on populations and the design of effective conservation evolving independently since an old allopatric fragmen- measures. We used mitochondrial DNA control region tation event. This result is also corroborated by cyto- and cytochrome b sequence data to investigate the spe- chrome b sequence data, for which marine and riverine specimens are fixed for haplotypes that differ by 28 (out Communicated by J. P. -

Fao/Government Cooperative Programme Scientific Basis
FI:GCP/RLA/140/JPN TECHNICAL DOCUMENT No. 3 FAO/GOVERNMENT COOPERATIVE PROGRAMME SCIENTIFIC BASIS FOR ECOSYSTEM-BASED MANAGEMENT IN THE LESSER ANTILLES INCLUDING INTERACTIONS WITH MARINE MAMMALS AND OTHER TOP PREDATORS CETACEAN SURVEYS IN THE LESSER ANTILLES 2000-2006 FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Barbados, 2008 FI:GCP/RLA/140/JPN TECHNICAL DOCUMENT No. 3 FAO/GOVERNMENT COOPERATIVE PROGRAMME SCIENTIFIC BASIS FOR ECOSYSTEM-BASED MANAGEMENT IN THE LESSER ANTILLES INCLUDING INTERACTIONS WITH MARINE MAMMALS AND OTHER TOP PREDATORS CETACEAN SURVEYS IN THE LESSER ANTILLES 2000-2006 Report prepared for the Lesser Antilles Pelagic Ecosystem Project (GCP/RLA/140/JPN) FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Barbados, 2008 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. -

Feeding Strategies of the Guiana Dolphin Sotalia Guianensis Marcos R
70 The Open Marine Biology Journal, 2009, 3, 70-76 Open Access Feeding Strategies of the Guiana Dolphin Sotalia guianensis Marcos R. Rossi-Santos*,1,§ and Paulo A.C. Flores2 1Centro de Ciências Biológicas, Universidade Federal de Santa Catarina and International Wildlife Coalition, Brasil 2Centro Nacional de Pesquisa, Conservação e Manejo de Mamíferos Aquáticos – CMA, ICMBio CMA SC – Rod. Maurício Sirotsky Sobrinho, s/n, km02, Jurerê, Florianópolis, SC, 88053-700, Brasil Abstract: The aim of this study was to characterize the feeding behavior of the Guiana dolphin, S. guianensis, through description and quantification of the strategies used by the dolphins, and possibly to apply this patterns to other areas in its distribution. Data were collected during systematic boat surveys, between November/1996 and May/1997, with 120 hours and 13 minutes of total observations. Scheffé Test was utilized to determine whether some strategies were used more than others. Six basic strategies of dolphin feeding behavior were identified: Individual Random Feeding (IRF), Group Random Feeding (GRF), Circular Cooperative Feeding (CCF), Front Cooperative feeding (FCF), Crossing Cooperative Feeding (CRF) and Zig-zag Cooperative Feeding (ZCF). The strategy GRF was statistically different from the others (Scheffé 5%). Previous studies suggested that S. guianensis exhibit two basic feeding strategies, while here we show greater diversity on the feeding behavior, with more complex and varied foraging strategies than previously reported for this species. Observed variation in the coordination of individuals, group cohesion, movement patterns, prey availability and environmental features, demonstrates the complexity of the S. guianensis feeding behavior. Keywords: Feeding behavior, Strategies, Guiana dolphin, Sotalia guianensis. -

Distribution and Movement Patterns of Killer Whales (Orcinus Orca) in the Northwest Atlantic. ICES CM 2008/B:20
ICES CM 2008/B:20 Distribution and Movement Patterns of Killer Whales (Orcinus orca) in the Northwest Atlantic Tara S. Stevens1,2 and Jack W. Lawson1 (1) Fisheries and Oceans Canada, NAFC, 80 East White Hills Road, St. John’s, Newfoundland and Labrador A1C 5X1, Canada (2) Memorial University of Newfoundland, Cognitive and Behavioural Ecology Program, St. John’s, Newfoundland and Labrador A1B 3X7, Canada Corresponding author e-mail: [email protected] ABSTRACT: Killer whales (Orcinus orca) occur throughout the northwest Atlantic. A sightings database and photographic catalogue, created mostly from opportunistic sources, is used to examine the occurrence of killer whales in Atlantic Canada. A majority of the sightings are from the Newfoundland and Labrador Region despite comparable observer coverage in adjacent areas such as the Gulf of St. Lawrence and Scotian Shelf, which suggests greater abundance in and habitat preference for Newfoundland and Labrador waters. Killer whales occur in all months of the year and in both near- and offshore regions, although particular sighting patterns may represent local observer effort and awareness. The distribution, movement, and residency patterns of killer whales may be closely linked to that of their prey; they have been observed harassing, attacking, and eating marine mammals, including minke whales (Balaenoptera acuterostrata), dolphins, and seals, and potentially eating fish. Some killer whales appear to remain year round in the Newfoundland and Labrador area and have been sighted during the spring within pack ice, potentially in association with breeding harp seals (Phoca groenlandica). Based on photographic records, individual killer whales in this area have been shown to move hundreds of kilometers within a year. -

Bottlenose Dolphins of North Patagonia
Dolphins of the Bay Discovering the bottlenose dolphins of North Patagonia ELS VERMEULEN . HILDA SUÁREZ . ALEJANDRO BALBIANO “We ourselves feel that what we are doing is just a drop in the ocean. But the ocean would be less because of that missing drop.” Mother Teresa of Calcutta (1910-1997) Table of Contents 2 Introduction Prologue Ever since I was a child, I have dreamed of working with 3 What is a cetacean? dolphins, my favourite animals. But it wasn’t until I was 20 4 Getting to know the dolphins that I saw my first wild dolphin. I will never forget it. It was a bottlenose dolphin, also known in Argentina as “tonina”. of the Gulf of San Matías The love I feel for these animals has cultivated the need to protect 5 The bottlenose dolphin them deep within me, seeking to ensure they are able to live in a healthy and peaceful environment. This passion motivates me 6 How do we study bottlenose dolphins? to learn about dolphins and study them in the wild, and is why 7 What do we know about the bottlenose I became a marine biologist. Besides, it is the perfect excuse to be around them all day long! Dolphins dolphins of the Bay of San Antonio? Studying the bottlenose dolphins in the Bay of San Antonio 8 Bottlenose dolphin tales I (Province of Río Negro, Argentina) has only deepened my passion further. During the years I have spent around these dolphins, not 9 Bottlenose dolphin tales II only have I begun to understand their life as a species, but of the Bay 10 Behaviour I have also begun to know each one of them individually, all with their different stories. -

Figure2 Taxonomic Revision of the Dolphin Genus Lagenorhynchus
A) LeDuc et al. 1999, Figure 1 - cyt b (1,140 bp) B) May-Collado & Agnarsson 2006, Figure 2 - cyt b (578 or 1,140 bp) C) Agnarsson & May-Collado 2008, Figure 5 - cyt b (578 or 1,140 bp) 100/100/34 100 100/100 Phocoena spp. Phocoenidae Phocoenidae 65/92 100/100/23 85 Monodontidae 100/100 Feresa attenuata 100 Monodontidae Monodontidae 58/79/1 94/92 Peponocephala electra Orcaella brevirostris 98/99/6 100 Cephalorhynchus commersonii Orcinus orca Globicephala spp. 97/97/9 98 Cephalorhynchus eutropia 100/100 Globicephala spp. Grampus griseus 51/57 80 Cephalorhynchus hectori 55/69 Peponocephala electra Pseudorca crassidens Cephalorhynchus heavisidii 100/100 58/77 Feresa attenuata Orcinus orca 100/100 96 Lagenorhynchus australis Grampus griseus 98/89/9 Orcaella sp. Lagenorhynchus cruciger Pseudorca crassidens 100 99 Lagenorhynchus obliquidens 100/100/13 Lissodelphis borealis 100/100 Cephalorhynchus commersonii Lissodelphis peronii Lagenorhynchus obscurus 99/99 Cephalorhynchus eutropia 56/69/1 Lagenorhynchus obscurus 100 Lissodelphis borealis 73/78 Cephalorhynchus hectori Lagenorhynchus obliquidens Lissodelphis peronii 64/62 Cephalorhynchus heavisidii 100/100/9 100/99/8 Lagenorhynchus cruciger 100 27/X 100/100 Lagenorhynchus australis Delphinus sp. 95/96 Lagenorhynchus australis Lagenorhynchus cruciger 98/99/12 99 Cephalorhynchus heavisidii 59 95 Stenella clymene 100/100 100/100 Lagenorhynchus obliquidens 63/57/ Lagenorhynchus obscurus 2 Cephalorhynchus hectori 100 Stenella coeruleoalba Cephalorhynchus eutropia Stenella frontalis 100/100 Lissodelphis borealis 98/99/5 Cephalorhynchus commersonii 57 Tursiops truncatus Lissodelphis peronii Lagenodelphis hosei Lagenorhynchus albirostris 100/100 100 Sousa chinensis Delphinus sp. Lagenorhynchus acutus 77/77 * Stenella attenuata 99/99 Stenella clymene Steno bredanensis 69 93/89 * Stenella longirostris Stenella coeruleoalba 37/X 99/99 Sotalia fluviatilis 68 51 Sotalia fluviatilis 100 100/100 Stenella frontalis Steno bredanensis Sousa chinensis 43/88 Tursiops aduncus 76/78/2 Lagenorhynchus acutus Tursiops truncatus Stenella spp.