Selecting and Propagating Clones of Bigtooth Maple (<I>Acer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mesa Glow Bigtooth Maple
HORTSCIENCE 53(5):734–736. 2018. https://doi.org/10.21273/HORTSCI12881-18 plant water relations, leaf relative water content (RWC), specific leaf weight, total Ò leaf area, specific stem length, leaf thickness, ‘JFS-NuMex 3’: Mesa Glow plant height, xylem diameter, leaf, stem, and root dry weight (DW), relative growth rate Bigtooth Maple (RGR), and net assimilation rate (NAR) in 1 plants exposed to multiple cycles of drought Rolston St. Hilaire compared with well-irrigated controls (Bsoul Department of Plant and Environmental Sciences, New Mexico State et al., 2006). A cycle of drought consisted of University, P.O. Box 30003, Las Cruces, NM 88003 irrigating plants only after pot gravimetric moisture loss because of evapotranspiration Additional index words. aceraceae, Acer grandidentatum, environmental stress, fall color, reached 56% to 57%. woody ornamentals Initial screening results revealed that se- lected provenances in Texas, New Mexico, and Utah might contain drought-tolerant Bigtooth maple (Acer grandidentatum more upright form, and redder fall colors than ecotypes (Bsoul et al., 2006). This prompted Nutt.) is a woody deciduous tree that is previous bigtooth maple selections. a second round of drought tolerance testing of indigenous only to North America (St. plants from those selected provenances in Hilaire, 2002). The plant has a contiguous Texas, New Mexico, and Utah in an outdoor ° Origin geographic range that covers 18 of latitude field setting from 23 Aug. to 11 Nov. 2003 and includes regions in Utah, Idaho, Wyom- Between 18 Aug. and 3 Nov. 2001, (Bsoul et al., 2007). On 30 Mar. 2003, plants ing, Arizona, New Mexico, and Texas (Bsoul mature samaras (seeds) of bigtooth maples were potted into 30-L pots using the same 1 et al., 2006). -

GREAT PLAINS REGION - NWPL 2016 FINAL RATINGS User Notes: 1) Plant Species Not Listed Are Considered UPL for Wetland Delineation Purposes
GREAT PLAINS REGION - NWPL 2016 FINAL RATINGS User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps region. -

Survival of Juvenile Acer Grandidentatum Nutt. (Bigtooth Maple, Aceraceae) in Central Texas Woodlands
American Journal of Plant Sciences, 2020, 11, 413-425 https://www.scirp.org/journal/ajps ISSN Online: 2158-2750 ISSN Print: 2158-2742 Survival of Juvenile Acer grandidentatum Nutt. (Bigtooth Maple, Aceraceae) in Central Texas Woodlands O. W. Van Auken1*, D. L. Taylor2 1Department of Environmental Science and Ecology, University of Texas at San Antonio, San Antonio, Texas, USA 2Cisebsi Ltd. Co., Fair Oaks Ranch, Texas, USA How to cite this paper: Van Auken, O.W. Abstract and Taylor, D.L. (2020) Survival of Juvenile Populations of Acer grandidentatum Nutt. (Bigtooth maple, Aceraceae = Sa- Acer grandidentatum Nutt. (Bigtooth Maple, Aceraceae) in Central Texas Woodlands. pindaceae) in central Texas are mostly found in isolated, deep, relatively re- American Journal of Plant Sciences, 11, mote, limestone canyons. Acer grandidentatum is found with a few other 413-425. mostly deciduous species. Recruitment of juveniles has been reported to be https://doi.org/10.4236/ajps.2020.113030 lacking. One population of A. grandidentatum juveniles was found in a li- mestone canyon in a State Natural Area in Central Texas. Fifty juveniles were Received: February 5, 2020 Accepted: March 23, 2020 located. Wire enclosures were placed around half of the seedlings with half Published: March 26, 2020 left in the open. In an adjacent canyon, 50 juvenile seedlings were planted in a similar habitat with adult A. grandidentatum trees nearby. Half were in en- Copyright © 2020 by author(s) and closures and half in the open. Plant survival was followed for four growing Scientific Research Publishing Inc. This work is licensed under the Creative seasons until November 2019. -

Maples in the Landscape Sheriden Hansen, Jaydee Gunnell, and Andra Emmertson
EXTENSION.USU.EDU Maples in the Landscape Sheriden Hansen, JayDee Gunnell, and Andra Emmertson Introduction Maple trees (Acer sp.) are a common fixture and beautiful addition to Utah landscapes. There are over one hundred species, each with numerous cultivars (cultivated varieties) that are native to both North America and much of Northern Europe. Trees vary in size and shape, from small, almost prostrate forms like certain Japanese maples (Acer palmatum) and shrubby bigtooth maples (Acer grandidentatum) to large and stately shade trees like the Norway maple (Acer platanoides). Tree shape can vary greatly, ranging from upright, columnar, rounded, pyramidal to spreading. Because trees come in a Figure 1. Severe iron chlorosis on maple. Note the range of shapes and sizes, there is almost always a interveinal chlorosis characterized by the yellow leaves spot in a landscape that can be enhanced by the and green veins. Spotting on the leaves is indicative of the addition of a maple. Maples can create a focal point beginning of tissue necrosis from a chronic lack of iron. and ornamental interest in the landscape, providing interesting textures and colors, and of course, shade. some micronutrients, particularly iron, to be less Fall colors typically range from yellow to bright red, available, making it difficult for certain trees to take adding a burst of color to the landscape late in the up needed nutrients. A common problem associated season. with maples in the Intermountain West is iron chlorosis (Figure 1). This nutrient deficiency causes Recommended Cultivars yellowing leaves (chlorosis) with green veins, and in extreme conditions, can cause death of leaf edges. -

Literature on the Vegetation of Oklahoma, 1964-1975
176 LITERATURE ON THE VEGETATION OF OKLAHOMA, 1964-1975 T. H. Milby Science Library, University of Oklahoma, Norman, Oklahoma Oklahoma vegetation has been studied extensively both in its role as a primary component of the environment and as it relates to animal life, geology, agriculture, and other aspects of the state's natural history and economy. These studies have resulted in a large and scattered body of literature. Two previous bibliographies have been published (371,389) dealing with Oklahoma vegetation, one in 1953 and the second in 1964. This third bibliography on Oklahoma vegetation is for the period 1964-1975 and adds 171 new titles to the two previous lists. Entries are arranged alphabetically by author and chronologically when more than one entry occurs by the same author(s). Numbering continues sequentially. Geographical regions used in the current list are the same as those used for the two precedent bibliographies and are illustrated by Fig. 1. Initials represent subdivisions within the state, except that OK is used to indicate papers dealing with the state as a whole and RE for those in which Oklahoma vegetation is considered as part of a larger region. Since knowledge of the vegetation of an area is often basic and preliminary to other research activities, the list includes a number of papers in which the main objective is to report findings other than vegetational analysis or description. The inclusion of this collateral material enhances the value of the list for plant ecologists as well as makes it useful for taxonomists, agronomists, geographers, zoologists, and other scientists. -

Plant Data Sheet Acer Grandidentatum (Bigtooth Maple)
Plant Data Sheet Acer grandidentatum (Bigtooth maple) www.maple-trees.com/ pages/canyon-maple.php Taxonomy: Family scientific name: Aceraceae Family common name: maples Genus: Acer Species: grandidentatum Species authority: L., Nutt. Variety: Acer grandidentatum var. grandidentatum Variety authority: Nutt Variety: Acer grandidentatum var. sinuosum Variety authority: (Rehd.) Little Sub-species: Cultivar: Authority for Sub-species: Common Name(s): bigtooth maple, canyon maple, sugar maple (5) Species Code: ACGR3 (8) General Information: General Distribution and Range: plants.usda.gov/ java/profile?symbol=ACGR3 http://content.answers.com/main/content/wp/en-commons/thumb/4/4b/240px-Map_genus_Acer.png Ecological Distribution: Has broad ecological amplitude but prefers sites in canyons, ravines, on lower slopes, and along mountain streams (9). Climate and elevation range: Most commonly found between 6,900 and 9,400 foot elevations (3). Prefers cool, moist sites but is tolerant to dry areas due to its ability to survive low water potentials (9). Local habitat, abundance, and commonly associated species: Commonly associated with Douglas-fir (Pseudotsuga menziesii), white fir (Abies concolor), narrowleaf cottonwood (Populus angustifolia) and ponderosa pine (Pinus ponderosa). Typically dominant in these plant communities (9). Plant strategy type / successional stage: Seedlings are shade tolerant and grow under sagebrush (Artemisia tridentate) canopy and Gambel oak. Bigtooth maple is an early to late successional species (9). Plant Characteristics: Tree-shrub lifeform (9). A deciduous tree that reaches 50 feet in height with a large canopy and grayish-brown bark. The leaves are 2 to 5 inches in diameter and are lobed and dark green (4). Propagation Details: Ecotype: not available Propogation Goal: plants Propogation Method: Usually propagated by seed or grafting onto sugar maple rootstock (10). -

Bird Communities of Gambel Oak: a Descriptive Analysis
United States Department of Agriculture Bird Communities Forest Service Rocky Mountain of Gambel Oak: A Research Station General Technical Report RMRS-GTR-48 Descriptive Analysis March 2000 Andreas Leidolf Michael L. Wolfe Rosemary L. Pendleton Abstract Leidolf, Andreas; Wolfe, Michael L.; Pendleton, Rosemary L. 2000. Bird communities of gambel oak: a descriptive analysis. Gen. Tech. Rep. RMRS-GTR-48. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 30 p. Gambel oak (Quercus gambelii Nutt.) covers 3.75 million hectares (9.3 million acres) of the western United States. This report synthesizes current knowledge on the composition, structure, and habitat relationships of gambel oak avian communities. It lists life history attributes of 183 bird species documented from gambel oak habitats of the western United States. Structural habitat attributes important to bird-habitat relationships are identified, based on 12 independent studies. This report also highlights species of special concern, provides recommendations for monitoring, and gives suggestions for management and future research. Keywords: Avian ecology, bird-habitat relationships, neotropical migrant, oakbrush, oak woodlands, scrub oak, Quercus gambelii, Western United States The Authors ______________________________________ Andreas Leidolf is a Graduate Research Assistant in the Department of Fisheries and Wildlife at Utah State University (USU). He received a B.S. degree in Forestry/Wildlife Management from Mississippi State University in 1995. He is currently completing his M.S. degree in Fisheries and Wildlife ecology at USU. Michael L. Wolfe is a Professor in the Department of Fisheries and Wildlife at USU. He received a B.S. degree in Wildlife Management at Cornell University in 1963 and his doctorate in Forestry/Wildlife Management at the University of Göttingen, Germany, in 1967. -

Download PCN-Acer-2017-Holdings.Pdf
PLANT COLLECTIONS NETWORK MULTI-INSTITUTIONAL ACER LIST 02/13/18 Institutional NameAccession no.Provenance* Quan Collection Id Loc.** Vouchered Plant Source Acer acuminatum Wall. ex D. Don MORRIS Acer acuminatum 1994-009 W 2 H&M 1822 1 No Quarryhill BG, Glen Ellen, CA QUARRYHILL Acer acuminatum 1993.039 W 4 H&M1822 1 Yes Acer acuminatum 1993.039 W 1 H&M1822 1 Yes Acer acuminatum 1993.039 W 1 H&M1822 1 Yes Acer acuminatum 1993.039 W 1 H&M1822 1 Yes Acer acuminatum 1993.076 W 2 H&M1858 1 No Acer acuminatum 1993.076 W 1 H&M1858 1 No Acer acuminatum 1993.139 W 1 H&M1921 1 No Acer acuminatum 1993.139 W 1 H&M1921 1 No UBCBG Acer acuminatum 1994-0490 W 1 HM.1858 0 Unk Sichuan Exp., Kew BG, Howick Arb., Quarry Hill ... Acer acuminatum 1994-0490 W 1 HM.1858 0 Unk Sichuan Exp., Kew BG, Howick Arb., Quarry Hill ... Acer acuminatum 1994-0490 W 1 HM.1858 0 Unk Sichuan Exp., Kew BG, Howick Arb., Quarry Hill ... UWBG Acer acuminatum 180-59 G 1 1 Yes National BG, Glasnevin Total of taxon 18 Acer albopurpurascens Hayata IUCN Red List Status: DD ATLANTA Acer albopurpurascens 20164176 G 1 2 No Crug Farm Nursery QUARRYHILL Acer albopurpurascens 2003.088 U 1 1 No Total of taxon 2 Acer amplum (Gee selection) DAWES Acer amplum (Gee selection) D2014-0117 G 1 1 No Gee Farms, Stockbridge, MI 49285 Total of taxon 1 Acer amplum 'Gold Coin' DAWES Acer amplum 'Gold Coin' D2015-0013 G 1 2 No Gee Farms, Stockbridge, MI 49285, USA Acer amplum 'Gold Coin' D2017-0075 G 2 2 No Shinn, Edward T., Wall Township, NJ 07719-9128 Total of taxon 3 Acer argutum Maxim. -

Annotated Checklist of Vascular Flora, Cedar Breaks National
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 ON THE COVER Peterson’s campion (Silene petersonii), Cedar Breaks National Monument, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 February 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. Current examples of such reports include the results of research that addresses natural resource management issues; natural resource inventory and monitoring activities; resource assessment reports; scientifi c literature reviews; and peer- reviewed proceedings of technical workshops, conferences, or symposia. -
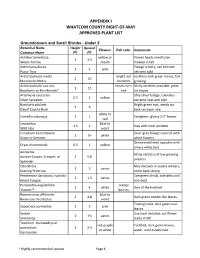
Whatcom County's Approved Plant List
APPENDIX I WHATCOM COUNTY RIGHT-OF-WAY APPROVED PLANT LIST Groundcovers and Small Shrubs - Under 2' Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Achillea tomentosa yellow or Flower heads need to be 1 3-5 Wooly Yarrow cream mowed in fall Antennaria dioica Foliage is furry, can tolerate 1 2 pink Pussy Toes extreme cold Arctostaphylos media bright red Leathery dark green leaves, fast 2 10 Manzanita Media branches growing Arctostaphylos uva ursi leaves turn Many varieties available, great 1 15 Bearberry or Kinnikinnick* red for slopes Artemesia caucasica Silky silver foliage, tolerates 0.5 2 yellow Silver Spreader extreme heat and cold Baccharis pilularis Bright green mat, needs cut 2 6 Dwarf Coyote Bush back once per year white to Camellia sasanqua 1 1 Evergreen, glossy 2-3" leaves red Ceanothus blue to 1.5 2 Stay with local varieties Wild Lilac violet Cerastium tomentosum Silver gray foliage covered with 1 5+ white Snow-in-Summer white flowers Ornamental seed capsules with Dryas drummondii 0.5 1 yellow silvery white tails Juniperus Many varieties of low growing Juniper-Carpet, Creeper, or 2 5-8 junipers Spreader Oenothera May die back in severe winters, 1 2 varies Evening Primrose come back strong Penstemon davidsonii, rupicola Evergreen shrub, tolerates cold 2 1.5 varies Beard Tongue and heat Pyracantha augustifolia orange 1 4 white One of the hardiest 'Gnome'* berries Rosmariunus officinalis blue to 2 4-8 Dark green needle-like leaves Rosemary 'Prostratus' violet Trailing habit, dark green oval Saponaria ocymoides 1 3 pink leaves Sedum Use local varieties, cut flower 2 1-5 varies Stonecrop stalks in fall Teucrium chamaedrys or red-purple Toothed, dark green leaves, postratium 1 2-3 or white water until established Germander * Highly-recommended species Page 1 Thymus white to 2 1-2 Many varieties Thyme purple Shrubs Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Arctostaphylos bright red Shiny dark green leaves turn 2-15 20 varies Manzanita berries maroon in fall. -

Species Replacement
Tree Taxonomy and Names Dr. Mike Kuhns USU Extension Forester What is taxonomy? • The practice and science of classification • Tree taxonomy – classifying trees botanically • Usually classify by anatomy, especially flowers and fruit; sometimes vascular, etc. • Often ecological similarities at family level and below Why do it? • Humans are classifiers • Knowing tree taxonomy let‟s you predict… – what tree will look like – how big it will get – how it will react to environment • The more precisely you can classify, the more precisely you can predict these things Maple Example • Maple – tells you little about appearance, drought hardiness, pH tolerance (opposite, lobed leaves; fruit samaras) Maple Example • Maple – tells you little about appearance, drought hardiness, pH tolerance (opposite, lobed leaves; fruit samaras) • Canyon maple (Acer grandidentatum) – drought & high pH tolerant, often good color, shape variable Maple Example • Maple – tells you little about appearance, drought hardiness, pH tolerance (opposite, lobed leaves; fruit samaras) • Canyon maple (Acer grandidentatum) – drought & high pH tolerant, often good color, shape variable • „Rocky Mountain Glow‟ canyon maple – so-so fall color, tree form Taxonomic Levels – Canyon Maple • Kingdom Plantae (Plants) Division Magnoliophyta (Angiosperms; flowering plants) Class Magnoliopsida (Dicotyledons) Subclass Rosidae (many orders; showy flowers) Order Sapindales (many families – citrus, cashew, etc.) Family Aceraceae (2 genera – Acer, Dipteronia) Genus Acer (120 species of maples) Species -

University Microfilms, Inc., Ann Arbor, Michigan the UNIVERSITY of OKLAHOMA
This dissertation has been microfihned exactly as received 69-5784 DENT, Thomas Curtis, 1928- RELATIONSHIPS OF TWO ISOLATED GROUPS OF SUGAR MAPLE IN CENTRAL OKLAHOMA TO EASTERN AND WESTERN SPECIES. The University of Oklahoma, Ph.D., 1969 Botany University Microfilms, Inc., Ann Arbor, Michigan THE UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE RELATIONSHIPS OF TWO ISOLATED GROUPS OF SUGAR MAPLE IN CENTRAL OKLAHOMA TO EASTERN AND WESTERN SPECIES A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillm ent of the requirements for the degree of DOCTOR OF PHILOSOPHY BY THOMAS CURTIS DENT Norman, Oklahoma 1969 RELATIONSHIPS OF TWO ISOLATED GROUPS OF SUGAR MAPLE CENTRAL OKLAHOMA TO EASTERN AND WESTERN SPECIES APPROVED BY /^DISSERTATION COMMITTEE • • ACKNOWLEDGEMENTS Recognition should be made of financial support derived in part from funds provided by National Science Summer Institute Programs for Secondary School Teachers, National Science Summer Fellowship Programs for Secondary School Teachers and National Science Summer Fellowships for Graduate Teaching Assistants. Many of the staff of the Botany and Microbiology Department of The University of Oklahoma, Norman, Oklahoma, have contributed to this research. Particular thanks are extended to Dr. Doyle E. Anderegg, former department chairman, for making the program administratively possible and those of the committee who criticized this dissertation. Special appreciation is extended to Dr. George J. Goodman, curator of the Bebb Herbarium who has been interested in the problem of the central Oklahoma sugar maples for many years. Much contained herein reflects ideas which he developed. Gratitude is expressed for the use of fa c ilitie s at Brigham Young University, Provo, Utah and for the sampling and other favors of Or.