To Download the AAC Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Second Circular Conference Colombia in the IYL 2015
SECOND ANNOUNCEMENT International Conference \Colombia in the International Year of Light" June 16 - 19, 2015 Bogot´a& Medell´ın,Colombia 1 http://indico.cern.ch/e/iyl2015colombiaconf• International Conference Colombia in the IYL Dear Colleagues, The International Conference Colombia in the International Year of Light (IYL- ColConf2015) will be held in Bogot´a,Colombia on 16-17 June 2015, and Medell´ın, Colombia on 18-19 June 2015. IYLColConf2015 is expected to bring together 500- 600 scientists, other professionals, and students engaged to research, development and applications of science and technology of light. You are invited to attend this conference and take part in the discussions about the \state of the art" in this field, in company of world-renowned scientists. Organizers and promoters of this conference include: The Universidad de los Andes (University of the Andes), Bogot´a;the Universidad Nacional de Colombia (National University of Colombia), Bogot´aand Medell´ın; the Universidad de Antioquia (University of Antioquia), Medell´ın; the Academia Colombiana de Ciencias Exactas, Fisicas y Naturales (Colombian Academy of Exact, Physical and Natural Sciences); Colombian research groups working on topics related to optical sciences, and academic programs at the undergraduate and graduate levels. We are honored to host IYLColConf2015 in Bogot´aand Medell´ın,two of the main cities of Colombia, in June 2015. We look forward to seeing you there. Sincerelly yours, On behalf of the Executive Committee, Prof. Jorge Mahecha. Institute of Physics, Universidad de Antioquia. page 2 of 27 http://indico.cern.ch/e/iyl2015colombiaconf• International Conference Colombia in the IYL Contents • IYLColConf2015 Committees 3 • General Information and Deadlines 5 • Registration and Support Policy 7 • Further Information 8 • Scientific Program 9 • Conference proceedings 25 • Travel and Transportation 25 • Hotels and Accommodations 25 • Miscellaneous 25 • Sponsors 27 IYLColConf2015 Committees International Advisory Committee Prof. -

Austria University of Vienna All Belgium Catholic University Of
Monash University - Exchange Partners Faculties Minimum GPA/WAM Austria University of Vienna All WAM 65 Belgium Catholic University of Leuven BusEco WAM 70 Brazil Pontifical Catholic University (PUC) of Rio de Janeiro All WAM 70 Brazil Universidade de Brasilia All WAM 70 Brazil State University of Campinas (UNICAMP) Brazil All WAM 70 Brazil University of Sao Paulo Arts WAM 70 Canada Bishop's University All WAM 70 Canada Carleton University All WAM 70 Canada HEC Montreal BusEco GPA 3.0 Canada Queen's University Arts, Science, Engineering, BusEco GPA 2.7 Canada Simon Fraser University All WAM 70 Canada University of British Columbia All except BusEco & Law WAM 85 Canada University of Ottawa BusEco WAM 70 Canada University of Waterloo All WAM 70 Canada York University All except BusEco & Law WAM 70 Canada Osgoode Hall Law School (York University) Law WAM 70 - previous 2 yrs Chile La Pontificia Universidad Catholic de Chile All WAM 70 Chile Universidad Diego Portales All WAM 70 Chile Universidad de Chile All WAM 70 China Peking University All WAM 60 China Shanghai Jiao Tong University All WAM 60 China Sichuan University All WAM 60 China Tsinghua University All WAM 60 China Nanjing University All WAM 60 China Fudan University All WAM 60 China Harbin Institute of Technology All WAM 60 China Sun Yat-Sen University All WAM 60 China Univeristy of Science and Technology of China All WAM 60 China Xi'an Jiaotong University All WAM 60 China Zhejinag University All WAM 60 Colombia University of Antioquia All WAM 70 Denmark Copenhagen Business School -

Welcoming a New Century • Focus on Hope • Seminarian Ministries
The Crossroads The Alumni Magazine for Theological College • Fall 2018 Welcoming a New Century • Focus on Hope • Seminarian Ministries Theological College | The National Seminary of The Catholic University of America A Letter from the Rector The Crossroads is published three times a year by the Office of Institutional Advance- ment of Theological College. It is distributed via non-profit mail to alumni, bishops, voca- S. SVL II PI tion directors, and friends of TC. R T A II N I W Rector M A E S Rev. Gerald D. McBrearity, P.S.S. (’73) S H I N M G Media & Promotions V L T L O I N Managing Editor G I S Suzanne Tanzi ✣ A Horizon of Hope Contributing Writers Jonathan Barahona • James Buttner Roger Schutz in his In order to prepare future priests who will be able to restore hope Liam Gallagher • Dr. Kathleen Galleher book, Living Today for and confidence amongst the people of God, Theological College is Rev. Matthew Gworek • Cornelia Hart Contents responsible for discerning the readiness of a priesthood candidate Alexandre Jiménez-Alcântara God, wrote, “During to enter the seminary and benefit from the formation program; Michael Kielor • Rev. Mark Morozowich the darkest periods of psychological tests and interviews, background checks, training Dr. John McCarthy • Justin Motes A Letter from the Rector ................................................................... 1 history, quite often a programs related to protecting the most vulnerable are all essential Mary Nauman • Jonathan Pham to the process of acceptance into Theological College. Once accept- Community News small number of men Michael Russo • Charles Silvas and women, scattered ed, every seminarian is accompanied by a spiritual director and a Cassidy Stinson Ordinations 2018 ........................................................................ -

Bibliometric Study in Support of Norway's Strategy for International Research Collaboration
Bibliometric Study in Support of Norway's Strategy for International Research Collaboration Final report Bibliometric Study in Support of Norway's Strategy for International Research Collaboration Final report © The Research Council of Norway 2014 The Research Council of Norway P.O.Box 2700 St. Hanshaugen N–0131 OSLO Telephone: +47 22 03 70 00 Telefax: +47 22 03 70 01 [email protected] www.rcn.no/english The report can be ordered at: www.forskningsradet.no/publikasjoner or green number telefax: +47 800 83 001 Design cover: Design et cetera AS Printing: 07 Gruppen/The Research Council of Norway Number of copies: 150 Oslo, March 2014 ISBN 978-82-12-03310-8 (print) ISBN 978-82-12-03311-5 (pdf) Bibliometric Study in Support of Norway’s Strategy for International Research Collaboration Final Report March 14, 2014 Authors Alexandre Beaudet David Campbell Grégoire Côté Stefanie Haustein Christian Lefebvre Guillaume Roberge Other contributors Philippe Deschamps Leroy Fife Isabelle Labrosse Rémi Lavoie Aurore Nicol Bastien St-Louis Lalonde Matthieu Voorons Contact information Grégoire Côté, Vice-President, Bibliometrics [email protected] Brussels | Montreal | Washington Bibliometric Study in Support of Norway’s Strategy for Final Report International Research Collaboration Contents Contents .................................................................................................................. i Tables ................................................................................................................... iii -
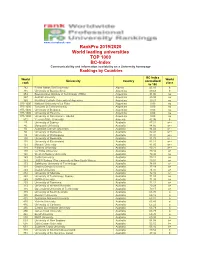
Rankpro 2019/2020 World Leading Universities TOP 1000 BC-Index Communicability and Information Availability on a University Homepage Rankings by Countries
www.cicerobook.com RankPro 2019/2020 World leading universities TOP 1000 BC-Index Communicability and information availability on a University homepage Rankings by Countries BC-Index World World University Country normalized rank class to 100 782 Ferhat Abbas Sétif University Algeria 45.16 b 715 University of Buenos Aires Argentina 49.68 b 953 Buenos Aires Institute of Technology (ITBA) Argentina 31.00 no 957 Austral University Argentina 29.38 no 969 Pontifical Catholic University of Argentina Argentina 20.21 no 975-1000 National University of La Plata Argentina 0.00 no 975-1000 Torcuato Di Tella University Argentina 0.00 no 975-1000 University of Belgrano Argentina 0.00 no 975-1000 University of Palermo Argentina 0.00 no 975-1000 University of San Andrés - UdeSA Argentina 0.00 no 812 Yerevan State University Armenia 42.96 b 27 University of Sydney Australia 87.31 a++ 46 Macquarie University Australia 84.66 a++ 56 Australian Catholic University Australia 84.02 a++ 80 University of Melbourne Australia 82.41 a++ 93 University of Wollongong Australia 81.97 a++ 100 University of Newcastle Australia 81.78 a++ 118 University of Queensland Australia 81.11 a++ 121 Monash University Australia 81.05 a++ 128 Flinders University Australia 80.21 a++ 140 La Trobe University Australia 79.42 a+ 140 Western Sydney University Australia 79.42 a+ 149 Curtin University Australia 79.11 a+ 149 UNSW Sydney (The University of New South Wales) Australia 79.01 a+ 173 Swinburne University of Technology Australia 78.03 a+ 191 Charles Darwin University Australia 77.19 -

Annual Report 2017
IDEAS LEADERSHIP ACTION OUR MISSION 2 Letter from Dan Porterfield, President and CEO WHAT WE DO 6 Policy Programs 16 Leadership Initiatives 20 Public Programs 26 Youth & Engagement Programs 30 Seminars 34 International Partnerships 38 Media Resources THE YEAR IN REVIEW 40 2017-2018 Selected Highlights of the Institute's Work 42 Live on the Aspen Stage INSTITUTIONAL ADVANCEMENT 46 Capital Campaigns 48 The Paepcke Society 48 The Heritage Society 50 Society of Fellows 51 Wye Fellows 52 Justice Circle and Arts Circle 55 Philanthropic Partners 56 Supporters STATEMENT OF FINANCIAL POSITION 90 2017 Annual Report WHO WE ARE 96 Our Locations 98 Aspen Institute Leadership 104 Board of Trustees LETTER FROM DAN PORTERFIELD, PRESIDENT AND CEO A LETTER FROM PRESIDENT AND CEO DAN PORTERFIELD There is nothing quite like the Aspen Institute. It is In the years to come, the Aspen Institute will deepen an extraordinary—and unique—American institution. our impacts. It is crucial that we enhance the devel- We work between fields and across divides as a opment of the young, address the urgent challenges non-profit force for good whose mission is to con- of the future, and renew the ideals of democratic so- vene change-makers of every type, established and ciety. I look forward to working closely with our many emerging, to frame and then solve society’s most partners and friends as we write the next chapter on important problems. We lead on almost every issue the Institute’s scope and leadership for America and with a tool kit stocked for solution-building—always the world. -

Calm Before the Storm - Q's Q's
Calm Before the Storm - Q's q's EXTRA ANSWERS (UNLOCKED, POST Confidence (Low, Mid, question # UPDATED Q QUESTIONS (CHRONOLOGICAL ORDER) ANSWERS (LOCKED) EXTRA ANSWERS (LOCKED) HERE) High) OCTOBER 29, 2017 According to Tripcode Q https://archive.4plebs.org/pol/thread/148790098#p148790685 LEGEND: SHORT, FACTUAL ANSWERS PLEASE, WITHOUT CONFIDENCE LEVEL WILL the below is his questions. MI - Miitary Intelligence TONS OF CONSPIRACY-LINKS/TIE-INS or NOW ONLY CAUSE THE DNI - Director of National Intelligence (also: ODNI) SOURCES SUCH AS ALEX JONES. SOURCES QUESTION NUMBER CELL TO https://archive.4plebs.org/pol/thread/148777785#p148782302 NSA - National Security Agency (US) NEED TO BE NORMIE-PALATABLE PLEASE CHANGE COLORS (INSTEAD IMAGE COMPILATION OF ALL OF Q'S VERIFIED POSTS IS ATTACHED TO THIS CIA - Central Intelligence Agency (US) OF ENTIRE ROW) FOR THE ROW. SIS - Secret Intelligence Service (also known as MI6) THIS IS FOR THE MEME MAGICIANS TO MAKE PEOPLE PRINTING THE -IT WILL BE KEPT UPDATED AS SOON AS A NEW ONE IS MADE AFTER MI5 - British Military Intelligence ?? FOR NORMIES. SHEET OUT TRIPCODE Q POSTING MI6 - British Military Intelligence ?? 1 https://img.4plebs.org/boards/pol/image/1510/46/1510468725387.jpg BO/BHO - Barak Hussein Obama draw.io HRC - Hillary Rodham Clinton WJC - William (Bill) Jefferson Clinton ANONS - PLEASE DON'T PUT ONLY PHOTOS IN DJT - Donald John Trump, US President THE OPEN COLUMNS, I'M UNABLE TO MOVE POTUS - President Of The United States THEM TO ANOTHER, EVEN IF THEY A JOHN M - John McCain, American Senator RELEVANT/GOOD. PLEASE POST A DIRECT LINK CF - Clinton Foundation WITH AN EXPLANATION OF WHAT IT IS _WITH_ THE PHOTO. -

Wake Forest College Faculty 1
Wake Forest College Faculty 1 Professor of Theatre WAKE FOREST COLLEGE BA, UNC-Chapel Hill; MFA, UNC-Greensboro. FACULTY Elizabeth Mazza Anthony (1998) Associate Teaching Professor of French Studies Date following name indicates year of appointment. Listings represent those BA, Duke University; MA, PhD, UNC-Chapel Hill. faculty teaching either full or part-time during the fall 2020 and/or spring Diana R. Arnett (2014) 2021. Associate Teaching Professor of Biology Irma V. Alarcón (2005) BS, MA, Youngstown State University; PhD, Kent State University. Associate Professor of Spanish and Italian Lisa Ashe (2016) BA, Universidad de Concepción (Chile); MA, PhD , Indiana University. Part-time Assistant Professor of Art Jane W. Albrecht (1987) BS, University of Tennessee; MA, PhD, University of Virginia. Professor of Spanish and Italian Miriam A. Ashley-Ross (1997) BA, Wright State University; MA, PhD , Indiana University. Professor of Biology Guillermo Alesandroni (2020) BS, Northern Arizona University; PhD, University of California (Irvine). Visiting Assistant Professor Robert J. Atchison (2010) BS, National University of Rosario; MS, University of Illinois at Chicago; Associate Professor of Communication and Director of Debate PhD, Oklahoma State University. BA, MA, Wake Forest University; PhD, University of Georgia. Rebecca W. Alexander (2000) Alison Atkins (2013) F.M. Kirby Family Faculty Fellow and Professor of Chemistry Assistant Teaching Professor of Spanish and Italian BS, Delaware; PhD, University of Pennsylvania. BA, Wake Forest University; MA, PhD , University of Virginia. Lucy Alfrd (2020) Emily A. Austin (2009) Assistant Professor of English Assistant Professor of Philosophy BA, University of Virginia; Phd, Stanford University; PhD, University of BA, Hendrix College; PhD, Washington University (St. -

Annual Report Occ Annual Report 2015
ORANGE COAST COLLEGE 2015 ANNUAL REPORT OCC ANNUAL REPORT 2015 MISSION STATEMENT Orange Coast College serves the educational needs of its diverse local and global community. The College empowers students to achieve their educational goals by providing high quality and innovative programs and services leading to academic degrees, college transfer, certificates in career and technical education, basic skills, and workforce development to enable lifelong learning. The college promotes student learning and development by fostering a respectful, supportive and participatory campus climate of student engagement and academic inquiry. 2 OCC ANNUAL REPORT 2014-15 ORANGE COAST COLLEGE ANNUAL REPORT OCC ANNUAL REPORT 2014-15 MESSAGE FROM THE PRESIDENT Dear Friends, These are some of the most exciting times in the history of Orange Coast College. In early November the Coast Community College District Board of Trustees unanimously voted to certify OCC’s final Environmental Impact Report (EIR) and approve the College’s vision 2020 Facilities Master Plan, paving the way for several highly anticipated buildings on our campus. Groundbreaking for OCC’s Planetarium is scheduled to take place in Summer 2016 (see page 9 for more details), and construction on a brand new OCC Recycling Center will begin in early May that expands on the popular community resource (page 10). Both of these facilities will positively impact not just our students, but also our surrounding community in meaningful ways that will be felt for many years to come, and we are immensely grateful for the support of our constituents. This past fall, OCC’s state-of-the-art Mathematics, Business and Computing Center opened to students — the 90,000-square-foot building houses 10 computing labs, three lecture halls and 30 faculty offices, and represents the caliber of new construction that we expect to see more of in the coming years. -

Javier Mejia Phone: +971 2628 66 92 | E-Mail: [email protected] | Web: Javiermejia.Strikingly.Com
Javier Mejia Phone: +971 2628 66 92 j E-mail: [email protected] j Web: javiermejia.strikingly.com Current employment Stanford University Palo Alto, US Postdoctoral Research Fellow 2021 { Present Education Los Andes University Bogota, Colombia Ph.D. in Economics (with honors) 2013 { 2018 Stanford University Palo Alto, US Visiting Student Researcher 2016 { 2017 Los Andes University Bogota, Colombia M.A. in Economics 2013 { 2015 University of Antioquia Medellin, Colombia B.A. in Economics 2007 { 2012 Research Experience Stanford University Palo Alto, US Postdoctoral Research Fellow 2021 { Present New York University Abu Dhabi Abu Dhabi, UAE Postdoctoral Associate 2018 { 2021 University of Bordeaux Bordeaux, France Visiting Research Fellow 2017 Los Andes University Bogota, Colombia Research Assistant 2013-2016 Teaching Experience Stanford University j Undergraduate level 2021-Present • Lecturer. Courses: Wealth of Nations, Societal Collapse New York University Abu Dhabi j Undergraduate level 2020-2021 • Lecturer. Courses: Wealth of Nations Los Andes University j Graduate and undergraduate level 2014-2016 • Adjunct professor. Courses: Principles of Mathematics • Teaching assistant. Courses: Advanced Macroeconomics, Economic History of Colombia Cooperative University of Colombia j Undergraduate level 2012-2013 • Adjunct professor. Courses: Economic Theory University Foundation of the Andean Region j Undergraduate level 2012-2013 • Adjunct professor. Courses: Economic Theory, Economic Policy Outreach Activity Forbes -Latin America- 2020 { -

WLU Table 2021
1000 WLU Matrix. 2021 World Leading Universities positions by the TOP and Rank groups TOP Country University Rank group 50 Australia Australian National University World Best 50 Australia University of Melbourne World Best 50 Australia University of Queensland World Best 50 Australia University of Sydney World Best 50 Canada McGill University World Best 50 Canada University of British Columbia World Best 50 Canada University of Toronto World Best 50 China Peking University World Best 50 China Tsinghua University World Best 50 Denmark University of Copenhagen World Best 50 Germany Heidelberg University World Best 50 Germany Ludwig-Maximilians University of Munich World Best 50 Germany Technical University Munich World Best 50 Hong Kong University of Hong Kong World Best 50 Japan Kyoto University World Best 50 Japan University of Tokyo World Best 50 Singapore National University of Singapore World Best 50 Switzerland EPFL Swiss Federal Institute of Technology Lausanne World Best 50 Switzerland ETH Zürich-Swiss Federal Institute of Technology Zurich World Best 50 Switzerland University of Zurich World Best 50 United Kingdom Imperial College London World Best 50 United Kingdom King's College London World Best 50 United Kingdom University College London World Best 50 United Kingdom University of Cambridge World Best 50 United Kingdom University of Edinburgh World Best 50 United Kingdom University of Manchester World Best 50 United Kingdom University of Oxford World Best 50 USA California Institute of Technology Caltech World Best 50 USA Carnegie -

Colombia – Student Activists – ESMAD – Universities
Refugee Review Tribunal AUSTRALIA RRT RESEARCH RESPONSE Research Response Number: COL33017 Country: Colombia Date: 16 April 2008 Keywords: Colombia – student activists – ESMAD – Universities This response was prepared by the Research & Information Services Section of the Refugee Review Tribunal (RRT) after researching publicly accessible information currently available to the RRT within time constraints. This response is not, and does not purport to be, conclusive as to the merit of any particular claim to refugee status or asylum. This research response may not, under any circumstance, be cited in a decision or any other document. Anyone wishing to use this information may only cite the primary source material contained herein. Questions 1. Please provide a description of an attack on protesters by the authorities (Anti- disturbance Mobile Squadrons or ESMAD) on 22 September 2005 at Valley University (Universidad del Valle – CERUV), including a list of the names of killed or wounded. Please include any eyewitness type reports with specific details. 2. Please provide information about ensuing, related protests, including about a protest at La Ermita church. 3. Please advise as to whether there was a national strike in Bogota on 22 May 2007? What was its purpose? 4. Please advise whether student activists were targeted in 2006 or 2007 by paramilitaries or the authorities? For what reasons were they targeted? Is there evidence they were seriously harmed? RESPONSE 1. Please provide a description of an attack on protesters by the authorities (Anti-disturbance Mobile Squadrons or ESMAD) on 22 September 2005 at Valley University (Universidad del Valle – CERUV), including a list of the names of killed or wounded.