A New Late Devonian Genus with Seed Plant Affinities Deming Wang* and Le Liu
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Study of the Primary Vascular System Of
Amer. J. Bot. 55(4): 464-472. 1!16'>. A COMPARATIVE STUDY OF THE PRIMARY VASCULAR SYSTE~1 OF CONIFERS. III. STELAR EVOLUTION IN GYMNOSPERMS 1 KADAMBARI K. NAMBOODIRI2 AND CHARLES B. BECK Department of Botany, University of Michigan, Ann Arbor ABST RAe T This paper includes a survey of the nature of the primary vascular system in a large number of extinct gymnosperms and progymnosperms. The vascular system of a majority of these plants resembles closely that of living conifers, being characterized, except in the most primitive forms which are protostelic, by a eustele consisting of axial sympodial bundles from which leaf traces diverge. The vascular supply to a leaf originates as a single trace with very few exceptions. It is proposed that the eustele in the gyrr.nosperms has evolved directly from the protostele by gradual medullation and concurrent separation of the peripheral conducting tissue into longitudinal sympodial bundles from which traces diverge radially. A subsequent modification results in divergence of traces in a tangential plane, The closed vascular system of conifers with opposite and whorled phyllotaxis, in which the vascular supply to a leaf originates as two traces which subsequently fuse, is considered to be derived from the open sympodial system characteristic of most gymnosperms. This hypothesis of stelar evolution is at variance with that of Jeffrey which suggests that the eustele of seed plants is derived by the lengthening and overlapping of leaf gaps in a siphonostele followed by further reduction in the resultant vascular bundles. This study suggests strongly that the "leaf gap" of conifers and other extant gymnosperms is not homologous with that of siphonostelic ferns and strengthens the validity of the view that Pterop sida is an unnatural group. -

S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

SYSTÉMATIQUE DES EMBRYOPHYTES Document Illustrant Plus Particulièrement Les Taxons Européens Version 4 Octobre 2007
Université Pierre et Marie Curie (PARIS 6) Préparation à l’agrégation SVSTU, secteur B SYSTÉMATIQUE DES EMBRYOPHYTES Document illustrant plus particulièrement les taxons européens version 4 octobre 2007 Catherine Reeb, Préparation agrégation SVSTU, UPMC Jean-Yves Dubuisson, Laboratoire Paléobotanique et Paléoécologie, équipe Paléodiversité, systématique et évolution des Embryophytes, UPMC Dessin de couverture : extrait d’un traité botanique tibétain Pour Garance Remerciements: merci à Jean et Monique Duperon pour les clés de détermination des familles d’Angiospermes, à A.M pour la reproduction de couverture, à Michaël Manuel et Eric Queinnec pour la partie I de l’introduction. Egalement à tous les illustrateurs qui ont autorisé l’utilisation de leurs documents mis en ligne sur Internet : Françoise Gantet, Prof. I. Foissner, Association Endemia de Nouvelle Calédonie. 1 Table des matières Introduction 3 Les données essentielles pour l’agrégation . 5 I Systématique générale des Embryophytes 6 I.1 Caractères des Embryophytes . 6 I.2 La place des Embryophytes au sein de la lignée verte . 7 I.3 Le groupe frère des Embryophytes . 7 I.4 Relations au sein des Embryophytes :les points acquis aujourd’hui . 8 I.4.1 Marchantiophytes, Bryophytes, Anthocérotophytes à la base des Embryophytes . 8 I.4.2 Les Trachéophytes ou plantes vasculaires, un groupe monophylétique . 9 I.4.3 Les Spermatophytes ou plantes à ovules, un groupe monophylétique . 9 I.5 Quelques points encore en discussion . 11 I.5.1 Position et relations des trois clades basaux (Bryophytes, Marchantiophytes et Anthocérotophytes) 11 I.5.2 Relations au sein des Spermatophytes :l’hypothèse "Gnepine" . 13 II Les principaux clades d’Embryophytes 15 II.1 MARCHANTIOPHYTA:Les Marchantiophytes ou Hépatiques . -

System Klasyfikacji Organizmów
1 SYSTEM KLASYFIKACJI ORGANIZMÓW Nie ma dziś ogólnie przyjętego systemu klasyfikacji organizmów. Nie udaje się osiągnąć zgodności nie tylko w odniesieniu do zakresów i rang poszczególnych taksonów, ale nawet co do zasad metodologicznych, na których klasyfikacja powinna być oparta. Zespołowe dzieła przeglądowe z reguły prezentują odmienne i wzajemnie sprzeczne podejścia poszczególnych autorów, bez nadziei na consensus. Nie ma więc mowy o skompilowaniu jednolitego schematu klasyfikacji z literatury i system przedstawiony poniżej nie daje nadziei na zaakceptowanie przez kogokolwiek poza kompilatorem. Przygotowany został w oparciu o kilka zasad (skądinąd bardzo kontrowersyjnych): (1) Identyfikowanie ga- tunków i ich klasyfikowanie w jednostki rodzajowe, rodzinowe czy rzędy jest zadaniem specjalistów i bez szcze- gółowych samodzielnych studiów nie można kwestionować wyników takich badań. (2) Podział świata żywego na królestwa, typy, gromady i rzędy jest natomiast domeną ewolucjonistów i dydaktyków. Powody, które posłużyły do wydzielenia jednostek powinny być jasno przedstawialne i zrozumiałe również dla niespecjalistów, albowiem (3) podstawowym zadaniem systematyki jest ułatwianie laikom i początkującym badaczom poruszanie się w obezwładniającej złożoności świata żywego. Wątpliwe jednak, by wystarczyło to do stworzenia zadowalającej klasyfikacji. W takiej sytuacji można jedynie przypomnieć, że lepszy ułomny system, niż żaden. Królestwo PROKARYOTA Chatton, 1938 DNA wyłącznie w postaci kolistej (genoforów), transkrypcja nie rozdzielona przestrzennie od translacji – rybosomy w tym samym przedzia- le komórki, co DNA. Oddział CYANOPHYTA Smith, 1938 (Myxophyta Cohn, 1875, Cyanobacteria Stanier, 1973) Stosunkowo duże komórki, dwuwarstwowa błona (Gram-ujemne), wewnętrzna warstwa mureinowa. Klasa CYANOPHYCEAE Sachs, 1874 Rząd Stigonematales Geitler, 1925; zigen – dziś Chlorofil a na pojedynczych tylakoidach. Nitkowate, rozgałęziające się, cytoplazmatyczne połączenia mię- Rząd Chroococcales Wettstein, 1924; 2,1 Ga – dziś dzy komórkami, miewają heterocysty. -

Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga
Subject BOTANY Paper No V Paper Code BOT521 Topic Taxonomy and Diversity of Seed Plant: Gymnosperms & Angiosperms Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants UNIT- I BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants Classification of Gymnosperms. # Robert Brown (1827) for the first time recognized Gymnosperm as a group distinct from angiosperm due to the presence of naked ovules. BENTHAM and HOOKSER (1862-1883) consider them equivalent to dicotyledons and monocotyledons and placed between these two groups of angiosperm. They recognized three classes of gymnosperm, Cyacadaceae, coniferac and gnetaceae. Later ENGLER (1889) created a group Gnikgoales to accommodate the genus giankgo. Van Tieghem (1898) treated Gymnosperm as one of the two subdivision of spermatophyte. To accommodate the fossil members three more classes- Pteridospermae, Cordaitales, and Bennettitales where created. Coulter and chamberlain (1919), Engler and Prantl (1926), Rendle (1926) and other considered Gymnosperm as a division of spermatophyta, Phanerogamia or Embryoptyta and they further divided them into seven orders: - i) Cycadofilicales ii) Cycadales iii) Bennettitales iv) Ginkgoales v) Coniferales vi) Corditales vii) Gnetales On the basis of wood structure steward (1919) divided Gymnosperm into two classes: - i) Manoxylic ii) Pycnoxylic The various classification of Gymnosperm proposed by various workers are as follows: - i) Sahni (1920): - He recognized two sub-divison in gymnosperm: - a) Phylospermae b) Stachyospermae BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants ii) Classification proposed by chamber lain (1934): - He divided Gymnosperm into two divisions: - a) Cycadophyta b) Coniterophyta iii) Classification proposed by Tippo (1942):- He considered Gymnosperm as a class of the sub- phylum pteropsida and divided them into two sub classes:- a) Cycadophyta b) Coniferophyta iv) D. -

Lyginopteris Oldhamia Class Cycadopsida Order Pteridospermales Family Lyginopteridaceae Genus Lyginopteris Species L.Oldhamia
Prepared for B.Sc. Part II Botany Hons. by Prof.(Dr.) Manorma Kumari Lyginopteris oldhamia Class Cycadopsida Order Pteridospermales Family Lyginopteridaceae Genus Lyginopteris Species L.oldhamia Lyginopteris is well known genus in the family Lyginopteridaceae. It was earlier known as Calymmatotheca hoeninghausii. Williamson, Scott, Brongniart, Binney, Potonie and Oliver and Scott described this genus in detail. It was found in abundance in the coal ball horizon of Lancashire and Yorkshire. Various parts of the plants were discovered and named separately. They were later found to be different organs of the same plants Lyginopteris due to presence of similar type of capitate gland on them (except roots). Various parts are: Leaves Sphenopteris hoeninghausii Stems Lyginopteris oldhamia Petioles Rachiopteris aspera Roots Kaloxylon hookeri Seeds Lagenostoma lomaxi Morphological Features The stem of Lyginopteris oldhamia was long aerial and radially symmetrical varying in diameter from 2mm to 4cm. It was frequently branched and bore adventitious roots that were probably prop roots that supported the weak stems. The leaves were spirally arranged and up to 0.5 metre long. The leaves were bipinnate to tripinnate. The pinnae had pinnules or leaflets and were present at right angles to the rachis. The rachis and petiole were studded with capitate glands. Lyginopteris oldhamia: A. Showing external character; B. Frond bearing pollen sac; C. Frond bearing seeds. 2 Anatomy Stem: The transverse sections of the stem show nearly circular outline. Outer layer is epidermis, next to it is many layered cortex. Outer cortex contains radially broadened fibrous strands that forms a vertical network. It provides mechanical strength to the weak stem. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Fundamentals of Palaeobotany Fundamentals of Palaeobotany cuGU .叮 v FimditLU'φL-EjAA ρummmm 吋 eαymGfr 伊拉ddd仇側向iep M d、 況 O C O W Illustrations by the author uc削 ∞叩N Nn凹創 刊,叫MH h 咀 可 白 a aEE-- EEA First published in 1987 by Chapman αndHallLtd 11 New Fetter Lane, London EC4P 4EE Published in the USA by Chα~pman and H all 29 West 35th Street: New Yo地 NY 10001 。 1987 S. V. M秒len Softcover reprint of the hardcover 1st edition 1987 ISBN-13: 978-94-010-7916-7 e-ISBN-13: 978-94-009-3151-0 DO1: 10.1007/978-94-009-3151-0 All rights reserved. No part of this book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage and retrieval system, without permission in writing from the publisher. British Library Cataloguing in Publication Data Mey凹, Sergei V. Fundamentals of palaeobotany. 1. Palaeobotany I. Title 11. Osnovy paleobotaniki. English 561 QE905 Library 01 Congress Catα loging in Publication Data Mey凹, Sergei Viktorovich. Fundamentals of palaeobotany. Bibliography: p. Includes index. 1. Paleobotany. I. Title. QE904.AIM45 561 8ι13000 Contents Foreword page xi Introduction xvii Acknowledgements xx Abbreviations xxi 1. Preservation 抄'pes αnd techniques of study of fossil plants 1 2. Principles of typology and of nomenclature of fossil plants 5 Parataxa and eutaxa S Taxa and characters 8 Peculiarity of the taxonomy and nomenclature of fossil plants 11 The binary (dual) system of fossil plants 12 The reasons for the inflation of generic na,mes 13 The species problem in palaeobotany lS The polytypic concept of the species 17 Assemblage-genera and assemblage-species 17 The cladistic methods 18 3. -
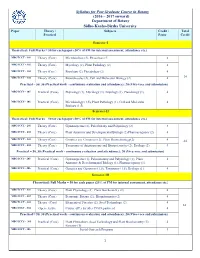
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

Syllabus M.Sc. Botany (Choice Based Credit System)
Syllabus M.Sc. Botany (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) DEPARTMENT OF BOTANY UNIVERSITY OF ALLAHABAD Page 1 of 21 DEPARTMENT OF BOTANY UNIVERSITY OF ALLAHABAD M.Sc. Syllabus (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) Semester – I Course Code Marks Course Title Credits BOT501 100 Phycology and Limnology 3 BOT502 100 Mycology and Plant Pathology 3 BOT503 100 Bryology and Pteridology 3 BOT504 100 Gymnosperm and Palaeobotany 3 BOT531 100 Lab Work I (based on Course BOT501 and BOT502) 4 (Excursion/field work/ Project) BOT532 100 Lab Work II (based on Course BOT503 and BOT504) 4 (Excursion/field work/Project) Total credits 20 Semester – II Course Code Marks Course Title Credits BOT505 100 Plant Morphology and Anatomy 3 BOT506 100 Reproductive Biology, Morphogenesis and Tissue culture 3 BOT507 100 Taxonomy of Angiosperm and Economic Botany 3 BOT508 100 Ecology and Phytogeography 3 BOT533 100 Lab Work III (based on Course BOT505 and BOT506) 4 (Field work/ Project) BOT534 100 Lab Work IV (based on Course BOT507 and BOT508) 4 (Field work/ Project) Total credits 20 Semester – III Course Code Marks Course Title Credits BOT601 100 Plant Physiology 3 BOT602 100 Plant Biochemistry and Biochemical Techniques 3 BOT603 100 Cytogenetics, Plant Breeding and Biostatistics 3 BOT604 100 Microbiology 3 BOT631 100 Lab Work V (based on Course BOT601 and BOT602) 4 BOT632 100 Lab Work VI (based on Course BOT603 and BOT604) 4 Total credits 20 Semester – IV Course Code Marks Course -

Palynology of the Permian Sanangoe
PALYNOLOGY OF THE PERMIAN SANANGOE- MEFIDEZI COAL BASIN,TETE PROVINCE, MOZAMBIQUE AND CORRELATIONS WITH GONDWANAN MICROFLORAL ASSEMBLAGES Kierra Caprice Mahabeer A Dissertation submitted to the Faculty of Science, University of the Witwatersrand, in partial fulfilment of the requirements for the Degree of Master of Science June, 2017 The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to the NRF. Declaration I hereby certify that this Dissertation is my own unaided work. It is being submitted for the degree of Master of Science at the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination at any other University. aT Kierra Caprice Mahabeer June 2017 n Acknowledgements My deepest gratitude goes to my supervisors Prof. Marion Bamford and Dr. Natasha Barbolini. Without their help and guidance this dissertation would not be possible. Thank you for always pushing me further and making me a better researcher. Thanks also goes to Dr. Jennifer Fitchett for the R script as well as for training in the statistical programs used in this study. Thank you to Eurasian Natural Resources Corporation (Mozambique Limitada) for access to core. Thank you to Dr. John Hancox for always being willing to assist with any questions and for facilitating access to core and for providing geological core logs. Thanks also goes to Prosper Bande for assistance with laboratory preparation. I am grateful for the financial assistance from the National Research Foundation the Palaeontological Scientific Trust (PAST) and the University of the Witwatersrand. -

BOSTONIA PERPLEXA GEN. ET SP. NOV., a CALAMOPITYAN AXIS from the NEW ALBANY SHALE of Kentucky1
Amer. J. Bot. 65(4): 459-465. 1978. BOSTONIA PERPLEXA GEN. ET SP. NOV., A CALAMOPITYAN AXIS FROM THE NEW ALBANY SHALE OF KENTUCKy1 WILLIAM E. STEIN, JR. AND CHARLES B. BECK Department of Botany. University of Michigan. Ann Arbor 48109 ABSTRACT Bostonia perplexa, gen. et sp. nov .. was collected from the Lower Mississippian Falling Run member of the Sanderson Formation. The single short segment of an axis. preserved as a petrifaction. contains at least three vascular columns. each with both primary and secondary tissues. Primary xylem is two or three ribbed. and contains several mesarch protoxylem strands. Gymnospermous secondary xylem is characterized by both uniseriate and multi seriate rays. The ground tissue is parenchymatous except for a few clusters of sclerotic cells. In its apparent polystelic nature. the specimen superficially resembles members of the Pennsylvanian to Permian Medullosaceae. All evi dence currently available. however. leads to the conclusion that this species should be placed in the Upper Devonian to Lower Mississippian Calamopityaceae. It has not been determined with certainty whether the species is polystelic (in the sense of the Medullosaceae), or whether the apparent polystely is the result of stelar branching proximal to the level of branch divergence. A SPECIMEN collected from the Falling Run among others. Reviews of the flora and stratig member of the Sanderson Formation and de raphy of the Devonian black shales were con scribed in this paper represents a new member tributed by Hoskins and Cross (195Ib) and Cross of the New Albany Shale flora. The vascular and Hoskins (1951). plant flora of the New Albany Shale is quite di As might be expected from its position in the verse, containing members from the Progymnos geologic column, the New Albany Shale flora permopsida, Lycopsida, Pteridospermales, Cla contains some elements typical of both the Up doxylales, and Coenopteridales.