Granny Smith
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Putative Cystathionine Beta-Synthase Homolog of Mycolicibacterium Smegmatis Is Involved in De Novo Cysteine Biosynthesis
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 5-2020 A Putative Cystathionine Beta-Synthase Homolog of Mycolicibacterium smegmatis is Involved in de novo Cysteine Biosynthesis Saroj Kumar Mahato University of Arkansas, Fayetteville Follow this and additional works at: https://scholarworks.uark.edu/etd Part of the Cell Biology Commons, Molecular Biology Commons, and the Pathogenic Microbiology Commons Citation Mahato, S. K. (2020). A Putative Cystathionine Beta-Synthase Homolog of Mycolicibacterium smegmatis is Involved in de novo Cysteine Biosynthesis. Theses and Dissertations Retrieved from https://scholarworks.uark.edu/etd/3639 This Thesis is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected]. A Putative Cystathionine Beta-Synthase Homolog of Mycolicibacterium smegmatis is Involved in de novo Cysteine Biosynthesis A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Cell and Molecular Biology by Saroj Kumar Mahato Purbanchal University Bachelor of Science in Biotechnology, 2016 May 2020 University of Arkansas This thesis is approved for recommendation to the Graduate Council. ___________________________________ Young Min Kwon, Ph.D. Thesis Director ___________________________________ ___________________________________ Suresh Thallapuranam, Ph.D. Inés Pinto, Ph.D. Committee Member Committee Member ABSTRACT Mycobacteria include serious pathogens of humans and animals. Mycolicibacterium smegmatis is a non-pathogenic model that is widely used to study core mycobacterial metabolism. This thesis explores mycobacterial pathways of cysteine biosynthesis by generating and study of genetic mutants of M. smegmatis. Published in vitro biochemical studies had revealed three independent routes to cysteine synthesis in mycobacteria involving separate homologs of cysteine synthase, namely CysK1, CysK2, and CysM. -

Organic Matter Composition Related to Methane Ebullitive Flux of an Urban Coastal Lagoon, Southeastern Brazil
Quim. Nova, Vol. 42, No. 6, 619-627, 2019 http://dx.doi.org/10.21577/0100-4042.20170373 ORGANIC MATTER COMPOSITION RELATED TO METHANE EBULLITIVE FLUX OF AN URBAN COASTAL LAGOON, SOUTHEASTERN BRAZIL Alessandra da Fonseca Vianaa,*, , Marco Aurélio dos Santosa, Marcelo Corrêa Bernardesb and Marcelo Amorima aInstituto Alberto Luiz Coimbra de Pós-Graduação e Pesquisa de Engenharia, Universidade Federal do Rio de Janeiro, 21941-450 Rio de Janeiro – RJ, Brasil b Departamento de Geoquímica, Universidade Federal Fluminense, 24020-141 Niterói – RJ, Brasil Artigo Recebido em 25/04/2019; aceito em 04/06/2019; publicado na web em 26/06/2019 In 2016, Brazil emitted 18.25 Tg of methane. Some of these emissions occur through continental aquatic ecosystems, locations of fate and accumulation of organic and inorganic matter. Thus, the aim of this study is to evaluate the origin of organic matter in Rodrigo de Freitas Lagoon, an eutrophic, coastal and chocked lagoon in Rio de Janeiro, Brazil, through the determination of n-alkanes and sterols compounds in the upper two centimeters of sediment and compare these data with methane ebullitive flux. The concentrations of n-alkanes varied between 2.43 and 25.82 µg g-1. C29 was predominant compound in most sites, but also with important petrogenic source evidenced by the occurrence of Unresolved Complex Misture. The total concentration of sterols ranged from 2.76 to 56.01 µg g-1. β-sitostanol was the most abundant compound and coprostanol was the most relevant at the sites under -2 -1 -2 -1 influence of domestic effluents. 4CH ebullitive flux averaged 199 mg m d in the dry period and 9.3 mg m d during the wet season. -

Biennial Review of 40 CFR Part 503 As Required Under the Clean Water Act Section 405(D)(2)(C)
United States Office of Water EPA-822R18003 Environmental Protection Agency Office of Science and Technology June 2019 4304T Biennial Review of 40 CFR Part 503 As Required Under the Clean Water Act Section 405(d)(2)(C) Biosolids Biennial Review Reporting Period 2016–2017 EPA-822R18003 Biennial Review of 40 CFR Part 503 As Required Under the Clean Water Act Section 405(d)(2)(C) Biosolids Biennial Review Reporting Period 2016–2017 U.S. Environmental Protection Agency Office of Water Office of Science and Technology Washington, D.C. June 2019 Biosolids Biennial Review Reporting Period 2016–2017 NOTICE This document has been reviewed in accordance with U.S. EPA policy and approved for publication. The report was prepared with the support of RTI International under the direction and review of the Office of Science and Technology. This document is not a regulation and does not change or substitute for statutory provisions and the EPA regulations. Thus, this document does not impose legally binding requirements on the EPA, states, tribes, or the regulated community. While the EPA has made every effort to ensure the accuracy of the discussion in this document, the obligations of the regulated community are determined by statutes, regulations, or other legally binding requirements. In the event of a conflict between the discussion in this document and any statute or regulation, this document would not be controlling. Mention of trade names or commercial products does not constitute endorsement or recommendation for their use. This document can be downloaded from the EPA’s website at http://www.epa.gov/biosolids. -

Proteome Cold-Shock Response in the Extremely Acidophilic Archaeon, Cuniculiplasma Divulgatum
microorganisms Article Proteome Cold-Shock Response in the Extremely Acidophilic Archaeon, Cuniculiplasma divulgatum Rafael Bargiela 1 , Karin Lanthaler 1,2, Colin M. Potter 1,2 , Manuel Ferrer 3 , Alexander F. Yakunin 1,2, Bela Paizs 1,2, Peter N. Golyshin 1,2 and Olga V. Golyshina 1,2,* 1 School of Natural Sciences, Bangor University, Deiniol Rd, Bangor LL57 2UW, UK; [email protected] (R.B.); [email protected] (K.L.); [email protected] (C.M.P.); [email protected] (A.F.Y.); [email protected] (B.P.); [email protected] (P.N.G.) 2 Centre for Environmental Biotechnology, Bangor University, Deiniol Rd, Bangor LL57 2UW, UK 3 Systems Biotechnology Group, Department of Applied Biocatalysis, CSIC—Institute of Catalysis, Marie Curie 2, 28049 Madrid, Spain; [email protected] * Correspondence: [email protected]; Tel.: +44-1248-388607; Fax: +44-1248-382569 Received: 27 April 2020; Accepted: 15 May 2020; Published: 19 May 2020 Abstract: The archaeon Cuniculiplasma divulgatum is ubiquitous in acidic environments with low-to-moderate temperatures. However, molecular mechanisms underlying its ability to thrive at lower temperatures remain unexplored. Using mass spectrometry (MS)-based proteomics, we analysed the effect of short-term (3 h) exposure to cold. The C. divulgatum genome encodes 2016 protein-coding genes, from which 819 proteins were identified in the cells grown under optimal conditions. In line with the peptidolytic lifestyle of C. divulgatum, its intracellular proteome revealed the abundance of proteases, ABC transporters and cytochrome C oxidase. From 747 quantifiable polypeptides, the levels of 582 proteins showed no change after the cold shock, whereas 104 proteins were upregulated suggesting that they might be contributing to cold adaptation. -

Chemical and Microbiological Water Quality of Subsurface Agricultural
In cooperation with the U.S. Department of Agriculture, Natural Resources Conservation Service and the Lenawee Conservation District Chemical and Microbiological Water Quality of Subsurface Agricultural Drains during a Field Trial of Liquid Dairy Manure Effluent Application Rate and Varying Tillage Practices, Upper Tiffin Watershed, Southeastern Michigan By Sheridan Kidd Haack and Joseph W. Duris Open-File Report 2008–1189 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia 2008 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested citation: Haack, S.K., and Duris, Joseph W., 2008, Chemical and microbiological water quality of subsurface agricultural drains during a field trial of liquid dairy manure effluent application rate and varying tillage practices, Upper Tiffin Watershed, southeastern Michigan: U.S. Geological Survey Open-File Report 2008– 1189, 38 p. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted -

Supplemental Methods
Supplemental Methods: Sample Collection Duplicate surface samples were collected from the Amazon River plume aboard the R/V Knorr in June 2010 (4 52.71’N, 51 21.59’W) during a period of high river discharge. The collection site (Station 10, 4° 52.71’N, 51° 21.59’W; S = 21.0; T = 29.6°C), located ~ 500 Km to the north of the Amazon River mouth, was characterized by the presence of coastal diatoms in the top 8 m of the water column. Sampling was conducted between 0700 and 0900 local time by gently impeller pumping (modified Rule 1800 submersible sump pump) surface water through 10 m of tygon tubing (3 cm) to the ship's deck where it then flowed through a 156 µm mesh into 20 L carboys. In the lab, cells were partitioned into two size fractions by sequential filtration (using a Masterflex peristaltic pump) of the pre-filtered seawater through a 2.0 µm pore-size, 142 mm diameter polycarbonate (PCTE) membrane filter (Sterlitech Corporation, Kent, CWA) and a 0.22 µm pore-size, 142 mm diameter Supor membrane filter (Pall, Port Washington, NY). Metagenomic and non-selective metatranscriptomic analyses were conducted on both pore-size filters; poly(A)-selected (eukaryote-dominated) metatranscriptomic analyses were conducted only on the larger pore-size filter (2.0 µm pore-size). All filters were immediately submerged in RNAlater (Applied Biosystems, Austin, TX) in sterile 50 mL conical tubes, incubated at room temperature overnight and then stored at -80oC until extraction. Filtration and stabilization of each sample was completed within 30 min of water collection. -

(12) United States Patent (10) Patent No.: US 7,906,710 B2 Karunanandaa Et Al
US00790671 OB2 (12) United States Patent (10) Patent No.: US 7,906,710 B2 Karunanandaa et al. (45) Date of Patent: *Mar. 15, 2011 (54) TRANSGENIC PLANTS CONTAINING FOREIGN PATENT DOCUMENTS ALTERED LEVELS OF STEROD EP 0486290 11, 1991 COMPOUNDS EP O480730 4f1992 JP O9121863 5, 1997 WO WO93,021.87 2, 1993 (75) Inventors: Balasulojini Karunanandaa, St. Louis, WO WO97/032O2 1, 1997 MO (US); Martha Post-Beittenmiller, WO WO97/34003 9, 1997 St. Louis, MO (US); Mylavarapu WO WO 98.45457 10, 1998 Venkatramesh, St. Louis, MO (US); WO WO99,04622 2, 1999 Ganesh M. Kishore, St. Louis, MO WO WOOOf 61771 10, 2000 (US); Gregory M. Thorne, St. Louis, WO WOO1/31027 3, 2001 MO (US); John R. LeDeaux, St. Louis, MO (US) OTHER PUBLICATIONS Bach et al., “Cloning of cDNAS or genes encoding enzymes of sterol (73) Assignee: Monsanto Company, St. Louis,MO biosynthesis from plants and other eukaryotes: heterologous expres (US) sion and complementation analysis of mutations for functional char acterization.” Progress in Lipid Research, 36(2/3): 197-226, 1997. (*) Notice: Subject to any disclaimer, the term of this Bak et al., “Cloning and expression in Escherichia coli of the patent is extended or adjusted under 35 obtusifoliol 14-alpha-demethylase of Sorghum bicolor (L.) Moench, U.S.C. 154(b) by 0 days. a cytochrome P450 orthologous to the sterol 14-alpha-demethylases This patent is Subject to a terminal dis (CYP51) from fungi and mammals.” Plant Journal, 11(2):191-201, claimer. 1997. Bak et al., “Cloning and expression in Escherichia coli of the obtusifoliol 14-alpha-demethylase of Sorghum bicolor (L.) Moench, (21) Appl. -

Structure of Soybean Serine Acetyltransferase
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 288, NO. 51, pp. 36463–36472, December 20, 2013 Published in the U.S.A. Structure of Soybean Serine Acetyltransferase and Formation of the Cysteine Regulatory Complex as a Molecular Chaperone* Received for publication, October 14, 2013, and in revised form, November 4, 2013 Published, JBC Papers in Press, November 13, 2013, DOI 10.1074/jbc.M113.527143 Hankuil Yi‡1, Sanghamitra Dey§1, Sangaralingam Kumaran¶, Soon Goo Leeʈ, Hari B. Krishnan**, and Joseph M. Jezʈ2 From the ‡Department of Biological Sciences, Chungnam National University, 220 Gung-Dong, Yuseong-Gu, Daejeon 305-764,Korea, the §Department of Biological Sciences, Presidency University, Kolkata, West Bengal 700073, India, the ¶Council of Scientific and Industrial Research, Institute of Microbial Technology, Sector 39-A, Chandigarh 160036, India, the ʈDepartment of Biology, Washington University, St. Louis, Missouri 63130, and the **Plant Genetics Research Unit, United States Department of Agriculture- Agricultural Research Service, Department of Agronomy, University of Missouri, Columbia, Missouri 65211 Background: Serine acetyltransferase (SAT) catalyzes the limiting step in cysteine biosynthesis. Results: Analysis of soybean SAT provides insight into catalysis and protein-protein interactions. Downloaded from Conclusion: Key structural features are required for catalysis and formation of a stable macromolecular complex. Significance: A new role for protein complex formation in plant cysteine biosynthesis is proposed. Serine acetyltransferase (SAT) catalyzes the limiting reaction sis plays a central role in fixing inorganic sulfur from the envi- in plant and microbial biosynthesis of cysteine. In addition to its ronment into the metabolic precursor for cellular thiol-con- http://www.jbc.org/ enzymatic function, SAT forms a macromolecular complex with taining compounds (3–6). -

Letters to Nature
letters to nature Received 7 July; accepted 21 September 1998. 26. Tronrud, D. E. Conjugate-direction minimization: an improved method for the re®nement of macromolecules. Acta Crystallogr. A 48, 912±916 (1992). 1. Dalbey, R. E., Lively, M. O., Bron, S. & van Dijl, J. M. The chemistry and enzymology of the type 1 27. Wolfe, P. B., Wickner, W. & Goodman, J. M. Sequence of the leader peptidase gene of Escherichia coli signal peptidases. Protein Sci. 6, 1129±1138 (1997). and the orientation of leader peptidase in the bacterial envelope. J. Biol. Chem. 258, 12073±12080 2. Kuo, D. W. et al. Escherichia coli leader peptidase: production of an active form lacking a requirement (1983). for detergent and development of peptide substrates. Arch. Biochem. Biophys. 303, 274±280 (1993). 28. Kraulis, P.G. Molscript: a program to produce both detailed and schematic plots of protein structures. 3. Tschantz, W. R. et al. Characterization of a soluble, catalytically active form of Escherichia coli leader J. Appl. Crystallogr. 24, 946±950 (1991). peptidase: requirement of detergent or phospholipid for optimal activity. Biochemistry 34, 3935±3941 29. Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and (1995). the thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281±296 (1991). 4. Allsop, A. E. et al.inAnti-Infectives, Recent Advances in Chemistry and Structure-Activity Relationships 30. Meritt, E. A. & Bacon, D. J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505± (eds Bently, P. H. & O'Hanlon, P. J.) 61±72 (R. Soc. Chem., Cambridge, 1997). -
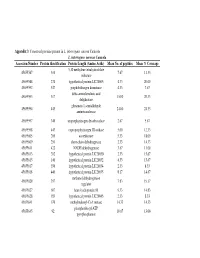
Appendix 3 and 4
Appendix 3 : Conserved proteins present in L. interrogans serovar Canicola L. interrogans serovar Canicola Accession Number Protein identification Protein Length (Amino Acids) Mean No. of peptides Mean % Coverage 5,10 methylene tetrahydrofolate 45655587 310 7.67 11.33 reductase 45655588 274 hypothetical protein LIC20005 4.33 20.00 45655592 547 porphobilinogen deaminase 4.33 7.67 delta-aminolevulinic acid 45655593 317 15.00 20.33 dehydratase glutamate-1-semialdehyde 45655594 443 24.00 24.33 aminotransferase 45655597 340 uroporphyrinogen decarboxylase 2.67 5.67 45655598 443 coproporphyrinogen III oxidase 5.00 12.33 45655605 200 azoreductase 5.33 18.00 45655609 251 short-chain dehydrogenase 2.33 14.33 45655611 422 NADH dehydrogenase 3.67 11.00 45655613 202 hypothetical protein LIC20030 2.33 15.67 45655615 140 hypothetical protein LIC20032 4.33 15.67 45655617 356 hypothetical protein LIC20034 2.33 8.33 45655618 440 hypothetical protein LIC20035 8.17 14.67 methanol dehydrogenase 45655620 357 7.83 19.17 regulator 45655627 607 heat shock protein 90 9.33 14.83 45655628 189 hypothetical protein LIC20045 2.33 8.33 45655641 670 methylmalonyl-CoA mutase 14.33 14.33 phosphoribosyl-ATP 45655645 92 10.67 13.00 pyrophosphatase 3-oxoacyl-(acyl-carrier protein) 45655647 254 4.00 17.00 reductase 45655648 77 acyl carrier protein 3.67 9.00 45655661 171 hypothetical protein LIC20078 5.00 26.00 4-hydroxybenzoyl-CoA 45655663 142 3.33 11.67 thioesterase S-adenosyl-L-homocysteine 45655666 436 13.00 19.67 hydrolase B12-dependent methionine 45655668 1247 42.67 21.67 -

Product Sheet Info
Product Information Sheet for NR-19703 Vibrio cholerae Gateway® Clone Set, Growth Conditions: Media: Recombinant in Escherichia coli, Plate 25 LB broth or agar containing 50 µg/mL kanamycin Incubation: Catalog No. NR-19703 Temperature: E. coli, strain DH10B-T1 clones should be This reagent is the tangible property of the U.S. Government. grown at 37°C. Atmosphere: Aerobic For research use only. Not for human use. Propagation: 1. Scrape top of frozen well with a pipette tip and streak onto Contributor: agar plate. Pathogen Functional Genomics Resource Center at the J. 2. Incubate the plates at 37°C for 1 day. Craig Venter Institute Citation: Manufacturer: Acknowledgment for publications should read “The following BEI Resources reagent was obtained through BEI Resources, NIAID, NIH: ® Vibrio cholerae Gateway Clone Set, Recombinant in Product Description: Escherichia coli, Plate 25, NR-19703.” Production in the 96-well format has increased risk of cross- contamination between adjacent wells. Individual clones Biosafety Level: 1 should be purified (e.g. single colony isolation and purification Appropriate safety procedures should always be used with this using good microbiological practices) and sequence-verified material. Laboratory safety is discussed in the following prior to use. BEI Resources does not confirm or validate publication: U.S. Department of Health and Human Services, individual mutants provided by the contributor. Public Health Service, Centers for Disease Control and Prevention, and National Institutes of Health. Biosafety in The Vibrio cholerae (V. cholerae) Gateway® clone set consists Microbiological and Biomedical Laboratories. 5th ed. of 46 plates which contain 3813 sequence validated clones Washington, DC: U.S. -

Phytosterols As Tracers of Terrestrial and Wetland Carbon to Ten Thousand Islands, Florida, USA
Phytosterols as tracers of terrestrial and wetland carbon to Ten Thousand Islands, Florida, USA: Implications for trophic resource use in the eastern oyster, Crassostrea virginica Derek J. Detweiler1, Ludovic Hermabessiere2, Patricia K. Goodman3, Aswani K. Volety1, Philippe Soudant2, Ai Ning Loh1 1 Center for Marine Science, University of North Carolina Wilmington 2 Laboratoire des Sciences de l’Environnement Marin, Institut Universitaire Européen de la Mer, Université de Bretagne Occidentale 3 Department of Marine and Ecological Sciences & Vester Field Station, Florida Gulf Coast University Background PRE-1900’s WATER FLOW Historic Flow Current Flow CERP Flow (U.S. Dept. Interior) Faka Union Canal (Everglades Foundation) CERP = Comprehensive Everglades Restoration Plan Rationale • The eastern oyster (Crassostrea virginica) • Distributed along Atlantic and Gulf coasts • Ecosystem service providers • Shoreline protection and stabilization • Water quality improvement • Substrate for mangrove propagation • “Canary in the coal mine” Condition indices for C. virginica in TTI oysters Loh et al. (2017) Volety et al. (2014) Rationale • Assessing oyster diet using phytosterols • Biochemicals in primary producers originating from C30 precursor • Well-preserved in estuarine environments • Determine organic matter sources – source specificity • Sterol proportions and configuration provides insight to community assemblages (uconn.edu) (sfsu.edu) (sms.si.edu) Bianchi and Canuel (2011) Objectives What is the pre-restoration impact on source contributions of terrestrial and aquatic organic matter as well as its quality and use as trophic resource? 1. Identify types and sources of sterols from POM, BMA, and oyster tissue. 2. Determine possible food source contributors to TTI. 3. Correlate biomarkers with food source quality. 4. Assess impact of altered freshwater discharge on C.