Microdochium Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3. Les Cyperaceae Et Les Poaceae
SECRÉTARIAT DE LA FAUNE ET DE LA FLORE COLLECTION PATRIMOINES NATIlRELS - VOLUME N° 11 Série Patrimoine Génétique /\r;~,v~ 1.1:: /tA "/~3 t\1 ~: -;1;\ rr.:'· ~~ ~!"'ir f" 1..:. S~'-,\; Gr2.f flt dlç G. CREMERS M. HOFF ~~1r~ 1r~@~@JNOI~~ ill)mW~1rm ill)~ ~ @lDJW~ W~~~~ ill[( = ILm CCW~~CC~rnTIr ILm W@~~~ MUSÉUM NATIONAL D'HISTOIRE NATURELLE PARIS. 1993 Cenchrus echinatus TI~Rr1J~~ 1J~@Rr@li\@1~~ J])~ JP~1J~ J])~ J1& @HIm~ W~(Ç~~~ mm = J1~ (ÇW~~(Ç~ rnTIr J1~ JP@&(Ç~ L'inventaire taxonomique des plantes de la Guyane française (troisième partie: Les Cypéracées et les Poacées) a été réalisé au : Laboratoire de botanique du Centre ORSTOM BP 165- F. 97323 Cayenne cedex avec le concours de la Direction Régionale de l'Environnement de Guyane (DIREN) cenchrus echina/us SECRÉTARIAT DE LA FAUNE ET DE LA FLORE COLLECTION PATRIMOINES NATIJRELS - VOLUME N° 11 Série Patrimoine Génétique G. CREMERS M. HOFF TI~~1r~ D~@~@EiOI~~ J])~IP~1r~ J])~ ~ @l1nf~ W~~~~~ TITITI = J1~ ~W~~~~ ~ J1~ IP@&~~ avec la collaboration de P. GOETGHEBEUR (Cypéracées) et E. JUDZIEWICZ (Poacées) CREMERS G. : Laboratoire de Botanique. Centre ORSTOM de CAYENNE. BP 165. F-97323 CAYENNE CEDEX. FRANCE GOETGHEBEUR P. : Laboratory ofPlant Morphology. Systematic and Ecology. University of Gent. Lederganckstraat 35. B-9000 GENr. BELGIUM HOFF M.: Laboratoire de Botanique. Centre ORSTOM de CAYENNE. BP 165. F-~7323 CAYENNE CEDEX. FRANCE JUDZIEWICZ E. : Smithsonian Institution. Department of Botany (NHB 166). WASHINGTON DC 20560. U.SA Les travaux de publications du SECRETARIAT DE LA FAUNE ETDE LA FWRE sont réalisés pour le compte du MINISTERE DE L'ENVIRONNEMENT DlRECI10N DE LA NATURE ET DES PAYSAGES MUSÉUM NATIONAL D'HISTOIRE NATURELLE PARIS, 1993 Edité par le SECRETARIAT DE LA FAUNE ET DE LA FWRE MUSEUM NATIONAL D'HISTOIRE NATURELLE SeIVice scientifique national associé par convention pennanente au MINISTERE DE L'ENVIRONNEMENT CETTE PUBLICATION CONSTITUE LE VOLUME Il DE LA COLLECTION PATRIMOINES NATURELS, Série Patrimoine Génétique. -

Weed Survey of Springvale Station
Weed Survey of Springvale Station Paul Williams Vegetation Management Science August 2016 1 Summary Springvale Station, approximately 50 km to the south-west of Cooktown, covers around 56,000 hectares. The property was purchased by the Queensland Government in May 2016 and is currently managed by the Queensland Department of Environment and Heritage Protection (EHP). A survey of weeds (i.e. invasive exotic plants) observable from the main vehicle tracks on Springvale Station was undertaken by Peter Munt, Keith Smith and Paul Williams on the 4 and 5 August 2016. The survey covered the northern half of the property and included inspections at points on the East, Granite and West Normanby Rivers. It also included surveys of two mine lease areas on the west Normanby River. At each record point, a GPS-derived location was documented and the abundance of all weeds in the surrounding area noted, based on an estimate of their percentage ground cover, grouped into categories (Table 1). Table 1. The abundance categories used for mapping the abundance of each weed (based on the categories used by Weeds of National Significance and the Queensland DPI). Weed Abundance Category Percentage cover of weed Scattered < 1 % cover Low 1 – 9 % cover Moderate 10 – 29 % cover High 30 – 49 % cover Very High ≥ 50 % cover Weed abundance was documented at regular intervals and where weed species composition or abundance changed. A total of 61 weeds were observed (Appendix 1). Nine of the weeds are declared as Category 3 under the new Queensland Biosecurity Act 2014. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
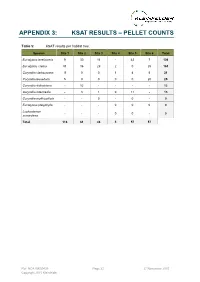
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential -

Floristic Composition of the South-Central Florida Dry Prairie Landscape Steve L
Floristic Composition of the South-Central Florida Dry Prairie Landscape Steve L. Orzell Avon Park Air Force Range, 29 South Blvd., Avon Park Air Force Range, FL 33825-5700 [email protected] Edwin L. Bridges Botanical and Ecological Consultant, 7752 Holly Tree Place NW, Bremerton, WA 98312-1063 [email protected] ABSTRACT Floristic composition of the Florida dry prairie landscape was compiled from 291 sites in nine south-central peninsular counties. Floristic lists were based upon field inventory and compilation from reliable sources to- taling 11,250 site and community type-specific observations and were analyzed by region (Kissimmee River, Desoto/Glades “Big Prairie,” and Myakka). The known vascular flora consists of 658 vascular plant taxa, rep- resenting 317 genera and 115 families. Families with the highest number of species are Poaceae (103), Asteraceae (78), Cyperaceae (76), Fabaceae (23), Scrophulariaceae (20), and Orchidaceae (18). The most diverse genera are Rhynchospora (29), Dichanthelium (17), Ludwigia (13), Xyris (12), and Andropogon (11). Of this flora 24 taxa are endemic to central or southern peninsular Florida, primarily within the pine savanna- flatwood/dry prairie landscape, and 41 taxa are of Floridian biotic affinity. Although most species are not re- gionally specific, a few (Carphephorus carnosus, Ctenium aromaticum, and Liatris spicata) appear to be ab- sent from the Myakka prairie region, while Marshallia tenuifolia appears to be absent from both the Desoto/ Glades and Myakka prairie regions. Within the dry prairie landscape Hypericum edisonianum is restricted to the Desoto/Glades region. A few other species somewhat differentiate between prairie regions; however, most occur in other habitats in the counties where they are absent or nearly absent from dry prairie. -

Schinus Molle L., Anacardiaceae) En México
POSGRADO EN CIENCIAS AMBIENTALES PROCESO DE INVASIÓN DEL PIRUL (SCHINUS MOLLE L., ANACARDIACEAE) EN MÉXICO Tesis que presenta M. EN C. JORGE ENRIQUE RAMÍREZ ALBORES Para obtener el grado de Doctor en Ciencias Ambientales Co-directores de tesis Dr. Ernesto Iván Badano, División de Ciencias Ambientales del Instituto Potosino de Investigación Científica y Tecnológica, A.C. Dr. Ramiro O. Bustamante, Instituto de Ecología y Biodiversidad y Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile. San Luis Potosí, México, Agosto de 2016 I II Créditos Institucionales Esta tesis fue elaborada en la División de Ciencias Ambientales del Instituto Potosino de Investigación Científica y Tecnológica, A.C., bajo la co-dirección del Dr. Ernesto Iván Badano. Durante sus estudios, el autor recibió una beca académica del Consejo Nacional de Ciencia y Tecnología (Beca # 120563). Este proyecto fue financiado mediante Recursos Propios del Instituto Potosino de Investigación Científica y Tecnológica, A.C., asignados al Dr. Ernesto I. Badano. III Dedicatoria A mi esposa Marlín por su amor, cariño, apoyo, paciencia, comprensión, consejos, sacrificios, y por todos los momentos que vivimos a lo largo de esta etapa…simplemente gracias por todo y por estar siempre a mi lado. A mi abuela Martha por su cariño y por darme siempre mucha fuerza para salir adelante. A mi mamá (Noemí, q.e.p.d) por ser mi motor, ejemplo de vida y por todas sus enseñanzas. A mis suegros Tomás y Elodia por su apoyo y cariño. A mi familia y amigos. V Agradecimientos Al Dr. Ernesto I. Badano por su paciencia, apoyo, consejos y amistad. -

Genus Sporobolus Was Agrostis
BLUMEA 35 (1991) 393-458 Sporobolus (Gramineae) in Malesia G.J. Baaijens & J.F. Veldkamp Rijksherbarium/Hortus Botanicus, Leiden, The Netherlands Summary R. Br. is 4 3 here In Malesia the genus Sporobolus (Gramineae) representedby sections, newly indicus has 5 1 and distinguished, with 10 species, 2 new. Sporobolus (L.) R. Br. varieties, new, var. (R. & Veldk., and var. 3 with a new rank: var. creber (De Nardi) Veldk., flaccidus S.) major Baaijens [S. creber De Nardi, S. diandrus (Retz.) Beauv., and S. fertilis (Steud.) Clayton, respective- S. laxus Simon from ly]. Some other non-Malesian taxa have also been reduced to varieties, e.g. Queensland (var. queenslandicus Veldk.), and S. pyramidalis Beauv. [var. pyramidalis (Beauv.) Veldk., incl. S. jacquemontii Kunth] from Africa and America. The Indian species generally known - S. as S. tremulus a superfluous name for S. virginicus (L.) Kunth -is reduced to a subspecies of humilis Presl. Sporobolus poiretii (R. & S.) Hitchc., long misapplied for S. indicus, is a synonym ofS. junceus (Beauv.) Kunth. Five new sections are distinguished. The correct name for Thysanolaena maxima (Roxb.) O. Ktze is T. latifolia (Roxb. ex Hornem.) Honda. Introduction The described R. Brown for three genus Sporobolus was by (1810) taxa repre- sented in his Australian collections which had previously been included in Agrostis close because is L. He regarded A. virginica L. as very (which was quite correct that but he retainedit in a species of Sporobolus as well), Agrostis, possibly because he had not seen any fruiting material (which is rare) and also because this species has exceptional long glumes. -

Group 2 – Inflorescence Digitate Or Subdigitate Paspalum *P
© James Cook University 2009 Grasses of James Cook University, http://eprints.jcu.edu.au/2104/ Townsville Campus Part B: Generic descriptions and key to Title: Grasses of James Cook University, Townsville campus. Part B: Generic descriptions and key to species [electronic resource] / N. B. Hooker. species ISBN: 9780980558623 (pdf) Notes: Includes index. Bibliography. Subjects: Grasses--Queensland--Identification. N.B. Hooker Other Authors/Contributors: James Cook University. School of Marine and Tropical Biology. COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of the James Cook University pursuant to Part VB of the Copyright Act 1968 (the Act). The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. School of Marine and Tropical Biology Do not remove this notice. James Cook University Townsville Drawings adapted from Mallett and Orchard (2002), Tothill and Hacker Queensland (1983), Wheeler et al. (2002), and drawn by N.B. Hooker. Photographs by C.P. Gardiner and taken from Sharp and Simon (2002). 1 Contents Acknowledgements Acknowledgements.....................................................2 I wish to thank the following people from James Cook Introduction .............................................................3 University for their help in the preparation of this book. Grasses listed according to habitat..................................4 Betsy Jackes who provided encouragement and support; her plant books1 were the major source of ideas and inspiration. Grass groups and genus information ................................5 Chris Gardiner, who is always willing to go on botanical walks Grass Group 1 ...........................................................6 around Townsville; he also loaned me his camera and provided photographs for the book. -

EL COMPLEJO SPOROBOLUS INDICUS (POACEAE, CHLORIDOIDEAE, ZOYSIEAE) EN LA ARGENTINA Darwiniana, Vol
Darwiniana ISSN: 0011-6793 [email protected] Instituto de Botánica Darwinion Argentina Denham, Silvia S.; Aliscioni, Sandra S. EL COMPLEJO SPOROBOLUS INDICUS (POACEAE, CHLORIDOIDEAE, ZOYSIEAE) EN LA ARGENTINA Darwiniana, vol. 49, núm. 1, 2011, pp. 32-42 Instituto de Botánica Darwinion Buenos Aires, Argentina Disponible en: http://www.redalyc.org/articulo.oa?id=66922015004 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto DARWINIANA 49(1): 32-42 2011 ISSN 0011-6793 EL COMPLEJO SPOROBOLUS INDICUS (POACEAE, CHLORIDOIDEAE, ZOYSIEAE) EN LA ARGENTINA Silvia S. Denham1 & Sandra S. Aliscioni2 1Instituto de Botánica Darwinion (ANCEFN-CONICET), Labardén 200, Casilla de Correo 22, B1642HYD San Isi- dro, Buenos Aires, Argentina; [email protected] (autor corresponsal). 2Cátedra de Botánica Agrícola, Facultad de Agronomía, Universidad de Buenos Aires, Av. San Martín 4453, C1417DSE Ciudad Autónoma de Buenos Aires, Argentina. Abstract. Denham, S. S. & S. S. Aliscioni. 2011. The Sporobolus indicus (Poaceae, Chloridoideae, Zoysieae) com- plex in Argentina. Darwiniana 49(1): 32-42. The species in the Sporobolus indicus complex are analyzed for Argentina. Sporobolus indicus var. indicus and S. indicus var. andinus are recognized for Argentina based on significant value differences in culms height and inflorescence length, and the altitude of collection area. The var. andinus is a new record for the Argentinean flora and it is here illustrated. Sporobolus jacquemontii is excluded from Argentina and S. -

Revisión De Las Especies Del Género Sporobolus
www.unal.edu.co/icn/publicaciones/caldasia.htm CaldasiaGiraldo-Cañas 31(1):41-76. & Peterson 2009 REVISIÓN DE LAS ESPECIES DEL GÉNERO SPOROBOLUS (POACEAE: CHLORIDOIDEAE: SPOROBOLINAE) DEL NOROESTE DE SUDAMÉRICA: PERÚ, ECUADOR, COLOMBIA Y VENEZUELA 1 Revision of the genus Sporobolus (Poaceae: Chloridoideae: Sporobolinae) for northwest South America: Peru, Ecuador, Colombia, and Venezuela DIEGO GIRALDO-CAÑAS Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado 7495, Bogotá D. C., Colombia. [email protected] PAUL M. PETERSON Department of Botany, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013-7012, U.S.A. [email protected] RESUMEN Se presenta un estudio taxonómico de las especies de Sporobolus para el noroeste de Sudamérica. Se reconocen once especies para el área de estudio. Se incluyen las claves para determinar las especies así como las descripciones morfológicas y sus ilustraciones. Se discuten para cada una de las especies sus relaciones morfológicas, su distribución geográfi ca y sus preferencias ecológicas; adicionalmente, se relacionan sus usos y sus nombres comunes. Asimismo, se propone la lectotipifi cación para Sporobolus lasiophyllus Pilg. Por otra parte, se registran tres especies por primera vez para Colombia [Sporobolus pilifer (Trin.)(Trin.) Kunth, Sporobolus tenuissimus (Mart. ex Schrank) Kuntze y Sporobolus virginicus (L.) Kunth]. Se excluyen de este tratamiento a Sporobolus brasiliensis (Raddi) Hack. (=EragrostisEragrostis airoidesairoides Nees) y Sporobolus domingensis (Trin.) Kunth. Adicionalmente, se propone la adopción y la unifi cación de algunos términos morfológicos en agrostología, tales como caña, panoja, espiguilla, lema, antecio y cariopsis, en lugar de culmo, panícula, espícula, lemma, fl ósculo y cariopse-cariópside, respectivamente. Palabras clave. -

Final Report to Queensland Department of Primary Industries & Fisheries
finalreportp NATURAL RESOURCE MANAGEMENT Project code: NBP.337 Prepared by: Dr Bill Palmer Biosecurity Queensland Department of Primary Industries and Fisheries, Queensland Date published: October 2008 ISBN: 978 1 74191 342 2 PUBLISHED BY Meat & Livestock Australia Limited Locked Bag 991 NORTH SYDNEY NSW 2059 Biological control of weedy sporobolus grasses by two host specific agents Meat & Livestock Australia acknowledges the matching funds provided by the Australian Government to support the research and development detailed in this publication. This publication is published by Meat & Livestock Australia Limited ABN 39 081 678 364 (MLA). Care is taken to ensure the accuracy of the information contained in this publication. However MLA cannot accept responsibility for the accuracy or completeness of the information or opinions contained in the publication. You should make your own enquiries before making decisions concerning your interests. Reproduction in whole or in part of this publication is prohibited without prior written consent of MLA. Biological control of weedy sporobolus grasses Abstract This report describes one of the first attempts at biological control of an invasive weedy grass. Five exotic Sporobolus spp., collectively known as the weedy sporobolus grasses, are serious weeds along the eastern seaboard of Australia. They are of extremely low palatability and cattle can not utilise them, and are also invasive and easily spread to new properties and areas. Biological control investigations commenced in 2000 with surveys of southern Africa, where S. pyramidalis, S. natalensis and S. africanus originate. Some 70 phytophagous insect species and 23 plant pathogens were found but only two organisms were considered potential biocontrol agents; the leaf smut Ustilago sporoboli-indici L. -

A Molecular Phylogeny of the Subtribe Sporobolinae and a Classification Of
A molecular phylogeny of the subtribe Sporobolinae and a classication of the subfamily Chloridoideae (Poaceae) P M. P1, 5, K R1, 2, Y H A3, J M. S4 1Smithsonian Institution, Department of Botany, National Museum of Natu ral History, Washington, DC 20013-7012, U.S.A.; email: peterson@si . edu 2M . G. Kholodny Institute of Botany, National Acad emy of Sciences, Kiev 01601, Ukraine; email: kromaschenko@gmail . com 3Instituto Politécnico Nacional, CIIDIR Unidad Durango- COFAA, Durango, C.P. 34220, Mexico; email: yolah54@gmail . com 4Canadian Museum of Nature, Research and Collections, Ottawa, Ontario K1P 6P4, Canada; email: [email protected] 5Author for correspondence; email: peterson@si . edu Abstract . The classication of the subtribe Sporobolinae containing the following six genera is poorly understood: Calamovilfa (ve species endemic to North Amer i ca), Crypsis (11 species en- demic to Asia and Africa), Psilolemma (one species endemic to Africa), Spartina (17 species cen- tered in North Amer i ca), Sporobolus (186 species distributed worldwide), and Thellungia (one species from Africa and Asia). The goal of this study was to reconstruct the evolutionary history of the Sporobolinae using molecular data with increased species sampling. Most species in this sub- tribe have spikelets with a single oret, one- veined (occasionally three- veined) lemmas, a ciliate membrane or line of hairs for a ligule, and fruits with free pericarps (modied caryopses). A phyloge ne tic analy sis was conducted on 161 species (250 samples), of which 134 species were in the Sporobolinae, using nuclear rITS (ribosomal internal transcribed spacer region) 1 and 2 sequences to infer evolutionary relationships. -

Agrostology; an Introduction to the Systematics of Grasses James P
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 2005 Agrostology; An Introduction to the Systematics of Grasses James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: http://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Agrostology; An Introduction to the Systematics of Grasses" (2005). Botanical Studies. 10. http://digitalcommons.humboldt.edu/botany_jps/10 This Grasses: General is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A G R O S T O L O G Y An Introduction to the Systematics of Grasses BY James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California Thirteenth Edition January 2005 Copyright 2005 by James P. Smith, Jr. Department of Biological Sciences Humboldt State University Arcata, California 95521 [email protected] All Rights Reserved No portion of this text may be reproduced in any form or by any electronic or mechanical means, including information storage and retrieval systems, without written permission. First Edition: 1972 Second Edition: 1973 Third Edition: 1976 Fourth Edition: 1978 Fifth Edition: 1979 Sixth Edition: 1983 Seventh Edition: 1994 Eighth Edition: 1995 Ninth Edition: 1996 Tenth Edition: 1998 Eleventh Edition: 2000 Twelfth Edition: 2002 T A B L E O F C O N T E N T S 1: INTRODUCTION .