Final Report to Queensland Department of Primary Industries & Fisheries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grass Genera in Townsville
Grass Genera in Townsville Nanette B. Hooker Photographs by Chris Gardiner SCHOOL OF MARINE and TROPICAL BIOLOGY JAMES COOK UNIVERSITY TOWNSVILLE QUEENSLAND James Cook University 2012 GRASSES OF THE TOWNSVILLE AREA Welcome to the grasses of the Townsville area. The genera covered in this treatment are those found in the lowland areas around Townsville as far north as Bluewater, south to Alligator Creek and west to the base of Hervey’s Range. Most of these genera will also be found in neighbouring areas although some genera not included may occur in specific habitats. The aim of this book is to provide a description of the grass genera as well as a list of species. The grasses belong to a very widespread and large family called the Poaceae. The original family name Gramineae is used in some publications, in Australia the preferred family name is Poaceae. It is one of the largest flowering plant families of the world, comprising more than 700 genera, and more than 10,000 species. In Australia there are over 1300 species including non-native grasses. In the Townsville area there are more than 220 grass species. The grasses have highly modified flowers arranged in a variety of ways. Because they are highly modified and specialized, there are also many new terms used to describe the various features. Hence there is a lot of terminology that chiefly applies to grasses, but some terms are used also in the sedge family. The basic unit of the grass inflorescence (The flowering part) is the spikelet. The spikelet consists of 1-2 basal glumes (bracts at the base) that subtend 1-many florets or flowers. -

Survey of Roadside Alien Plants in Hawai`I Volcanoes National Park and Adjacent Residential Areas 2001–2005
Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANts IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 Linda W. Pratt1 Keali`i F. Bio2 James D. Jacobi1 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kilauea Field Station, P.O. Box 44, Hawaii National Park, HI 96718 2 Hawai‘i Cooperative Studies Unit, University of Hawai‘i at Hilo, P.O. Box 44, Hawai‘i National Park, HI 96718 Hawai‘i Cooperative Studies Unit University of Hawai‘i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This product was prepared under Cooperative Agreement CA03WRAG0036 for the Pacific Island Ecosystems Research Center of the U.S. Geological Survey. Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANTS IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 1 2 1 LINDA W. PRATT , KEALI`I F. BIO , AND JAMES D. JACOBI 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kīlauea Field Station, P.O. Box 44, Hawai`i Volcanoes National Park, HI 96718 2 Hawaii Cooperative Studies Unit, University of Hawai`i at Hilo, Hilo, HI 96720 Hawai`i Cooperative Studies Unit University of Hawai`i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This article has been peer reviewed and approved for publication consistent with USGS Fundamental Science Practices ( http://pubs.usgs.gov/circ/1367/ ). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. -

Session 3: Non-Traditional Biological Control Agents 102 Session 3 Non-Traditional Biological Control Agents
Session 3: Non-Traditional Biological Control Agents 102 Session 3 Non-Traditional Biological Control Agents XIII International Symposium on Biological Control of Weeds - 2011 Session 3 Non-Traditional Biological Control Agents 103 The Case for Biological Control of Exotic African Grasses in Australia and USA Using Introduced Detritivores D. Sands1 and J. A. Goolsby2 1 CSIRO Division of Ecosystem Sciences, PO box 2583, Brisbane, Queensland 4001 Australia Email: [email protected] 2 U.S. Department of Agriculture-Agricultural Research Service (USDA-ARS), Beneficial Insects Research Unit, 2413 E Hwy 83, Weslaco, Texas 78596 USA Email: [email protected] Abstract Many species of African grasses were introduced into Australia and the USA to improve the quality and biomass of green pastures for domestic livestock. However, a range of non-target environmental impacts eventuated from these introductions, including spatial displacement of indigenous ecosystems, and induced changes to soils and nutrient re-cycling. The accumulation in biomass of flammable and senescing grasses predisposes grassland and woodland ecosystems to increasing impacts from wildfires, threatening indigenous plants and animals, and the recovery after fires of natural ecosystems. Very few detritivores have adapted to feed on and decompose detritus from African grasses in Australia and the USA, resulting in accumulation of dead leaves and a build-up of fuel increasing the risks from wildfires. In grasslands and woodlands of the Northern Hemisphere, epigeic detritivores and leaf-shredders include groups of invertebrates such as earthworms and isopods. In Australia, larvae of leaf-shredding moths, beetles and several other insects are important detritivores including oecophorid moths, cryptocephaline beetles, termites and cockroaches; some specifically adapted to breakdown of sub-surface plant materials in dry and moist ecosystems. -

Distribution and Diversity of Grass Species in Banni Grassland, Kachchh District, Gujarat, India
Patel Yatin et al. IJSRR 2012, 1(1), 43-56 Research article Available online www.ijsrr.org ISSN: 2279-0543 International Journal of Scientific Research and Reviews Distribution and Diversity of Grass Species in Banni Grassland, Kachchh District, Gujarat, India 1* 2 3 Patel Yatin , Dabgar YB , and Joshi PN 1Shri Jagdish Prasad Jhabarmal Tibrewala University, Jhunjhunu, Rajasthan 2R.R. Mehta Colg. of Sci. and C.L. Parikh College of Commerce, Palanpur, Banaskantha, Gujarat. 3Sahjeevan, 175-Jalaram Society, Vijay Nagar, Bhuj- Kachchh, Gujarat ABSTRACT: Banni, an internationally recognized unique grassland stretch of Western India. It is a predominantly flat land with several shallow depressions, which act as seasonal wetlands after monsoon and during winter its converts into sedge mixed grassland, an ideal dual ecosystem. An attempt was made to document ecology, biomass and community based assessment of grasses in Banni, we surveyed systematically and recorded a total of 49 herbaceous plant species, being used as fodder by livestock. In which, the maximum numbers of species (21 Nos.) were recorded in Echinocloa and Cressa habitat; followed by Sporobolus and Elussine habitat (20 species); and Desmostechia-Aeluropus and Cressa habiat (19 species). A total of 21 highest palatable species were recorded in Echinocloa-Cressa communities followed by Sporobolus-Elussine–Desmostechia (20 species and 18 palatable species) and Aeluropus–Cressa (19 species and 17 palatable species). For long-term conservation of Banni grassland, we also suggest a participatory co-management plan. KEY WORDS: Banni, Grassland, Palatability, Communities, Conservation. *Corresponding Author: Yatin Patel Research Scholar, Shri Jagdish Prasad Jhabarmal Tibrewala University, Jhunjhunu, Rajasthan E-mail: [email protected] IJSRR 1(1) APRIL-JUNE 2012 Page 43 Patel Yatin et al. -

3. Les Cyperaceae Et Les Poaceae
SECRÉTARIAT DE LA FAUNE ET DE LA FLORE COLLECTION PATRIMOINES NATIlRELS - VOLUME N° 11 Série Patrimoine Génétique /\r;~,v~ 1.1:: /tA "/~3 t\1 ~: -;1;\ rr.:'· ~~ ~!"'ir f" 1..:. S~'-,\; Gr2.f flt dlç G. CREMERS M. HOFF ~~1r~ 1r~@~@JNOI~~ ill)mW~1rm ill)~ ~ @lDJW~ W~~~~ ill[( = ILm CCW~~CC~rnTIr ILm W@~~~ MUSÉUM NATIONAL D'HISTOIRE NATURELLE PARIS. 1993 Cenchrus echinatus TI~Rr1J~~ 1J~@Rr@li\@1~~ J])~ JP~1J~ J])~ J1& @HIm~ W~(Ç~~~ mm = J1~ (ÇW~~(Ç~ rnTIr J1~ JP@&(Ç~ L'inventaire taxonomique des plantes de la Guyane française (troisième partie: Les Cypéracées et les Poacées) a été réalisé au : Laboratoire de botanique du Centre ORSTOM BP 165- F. 97323 Cayenne cedex avec le concours de la Direction Régionale de l'Environnement de Guyane (DIREN) cenchrus echina/us SECRÉTARIAT DE LA FAUNE ET DE LA FLORE COLLECTION PATRIMOINES NATIJRELS - VOLUME N° 11 Série Patrimoine Génétique G. CREMERS M. HOFF TI~~1r~ D~@~@EiOI~~ J])~IP~1r~ J])~ ~ @l1nf~ W~~~~~ TITITI = J1~ ~W~~~~ ~ J1~ IP@&~~ avec la collaboration de P. GOETGHEBEUR (Cypéracées) et E. JUDZIEWICZ (Poacées) CREMERS G. : Laboratoire de Botanique. Centre ORSTOM de CAYENNE. BP 165. F-97323 CAYENNE CEDEX. FRANCE GOETGHEBEUR P. : Laboratory ofPlant Morphology. Systematic and Ecology. University of Gent. Lederganckstraat 35. B-9000 GENr. BELGIUM HOFF M.: Laboratoire de Botanique. Centre ORSTOM de CAYENNE. BP 165. F-~7323 CAYENNE CEDEX. FRANCE JUDZIEWICZ E. : Smithsonian Institution. Department of Botany (NHB 166). WASHINGTON DC 20560. U.SA Les travaux de publications du SECRETARIAT DE LA FAUNE ETDE LA FWRE sont réalisés pour le compte du MINISTERE DE L'ENVIRONNEMENT DlRECI10N DE LA NATURE ET DES PAYSAGES MUSÉUM NATIONAL D'HISTOIRE NATURELLE PARIS, 1993 Edité par le SECRETARIAT DE LA FAUNE ET DE LA FWRE MUSEUM NATIONAL D'HISTOIRE NATURELLE SeIVice scientifique national associé par convention pennanente au MINISTERE DE L'ENVIRONNEMENT CETTE PUBLICATION CONSTITUE LE VOLUME Il DE LA COLLECTION PATRIMOINES NATURELS, Série Patrimoine Génétique. -

Final Repport
final repport Project code: B.NBP.0716 Prepared by: Ann Lawrie Royal Melbourne Institute of Technology Date published: February 2014 PUBLISHED BY Meat & Livestock Australia Limited Locked Bag 991 NORTH SYDNEY NSW 2059 Giant Rat’s Tail grass susceptibility to fungi effective in biological control of Giant Parramatta Grass Meat & Livestock Australia acknowledges the matching funds provided by the Australian Government to support the research and development detailed in this publication. This publication is published by Meat & Livestock Australia Limited ABN 39 081 678 364 (MLA). Care is taken to ensure the accuracy of the information contained in this publication. However MLA cannot accept responsibility for the accuracy or completeness of the information or opinions contained in the publication. You should make your own enquiries before making decisions concerning your interests. Reproduction in whole or in part of this publication is prohibited without prior written consent of MLA. Giant Rat's Tail grass susceptibility to fungi effective in biological control of Giant Parramatta Grass __________________________________________________________________________________ Abstract Sporobolus pyramidalis (Giant Rat’s Tail grass - GRT) was tested in seedling and pot trials to see if inoculation with Nigrospora oryzae and Fusarium sp. would produce the same symptoms in GRT as those observed in S. fertilis (Giant Parramatta Grass - GPG) – blight in pot trials and crown rot in the field. Seed germination was maximal at over 90% with surface-sterilisation and alternating temperatures of 15/35°C. In seedling trials in Petri dishes, inoculation with N. oryzae significantly reduced height but there was no chlorosis of the youngest leaf or mortality, whereas inoculation with Fusarium proliferatum or with both fungi killed the seedlings. -

A Preliminary List of the Vascular Plants and Wildlife at the Village Of
A Floristic Evaluation of the Natural Plant Communities and Grounds Occurring at The Key West Botanical Garden, Stock Island, Monroe County, Florida Steven W. Woodmansee [email protected] January 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to CarolAnn Sharkey Key West Botanical Garden 5210 College Road Key West, Florida 33040 and Kate Marks Heritage Preservation 1012 14th Street, NW, Suite 1200 Washington DC 20005 Introduction The Key West Botanical Garden (KWBG) is located at 5210 College Road on Stock Island, Monroe County, Florida. It is a 7.5 acre conservation area, owned by the City of Key West. The KWBG requested that The Institute for Regional Conservation (IRC) conduct a floristic evaluation of its natural areas and grounds and to provide recommendations. Study Design On August 9-10, 2005 an inventory of all vascular plants was conducted at the KWBG. All areas of the KWBG were visited, including the newly acquired property to the south. Special attention was paid toward the remnant natural habitats. A preliminary plant list was established. Plant taxonomy generally follows Wunderlin (1998) and Bailey et al. (1976). Results Five distinct habitats were recorded for the KWBG. Two of which are human altered and are artificial being classified as developed upland and modified wetland. In addition, three natural habitats are found at the KWBG. They are coastal berm (here termed buttonwood hammock), rockland hammock, and tidal swamp habitats. Developed and Modified Habitats Garden and Developed Upland Areas The developed upland portions include the maintained garden areas as well as the cleared parking areas, building edges, and paths. -

Large Trees, Supertrees and the Grass Phylogeny
LARGE TREES, SUPERTREES AND THE GRASS PHYLOGENY Thesis submitted to the University of Dublin, Trinity College for the Degree of Doctor of Philosophy (Ph.D.) by Nicolas Salamin Department of Botany University of Dublin, Trinity College 2002 Research conducted under the supervision of Dr. Trevor R. Hodkinson Department of Botany, University of Dublin, Trinity College Dr. Vincent Savolainen Jodrell Laboratory, Molecular Systematics Section, Royal Botanic Gardens, Kew, London DECLARATION I thereby certify that this thesis has not been submitted as an exercise for a degree at any other University. This thesis contains research based on my own work, except where otherwise stated. I grant full permission to the Library of Trinity College to lend or copy this thesis upon request. SIGNED: ACKNOWLEDGMENTS I wish to thank Trevor Hodkinson and Vincent Savolainen for all the encouragement they gave me during the last three years. They provided very useful advice on scientific papers, presentation lectures and all aspects of the supervision of this thesis. It has been a great experience to work in Ireland, and I am especially grateful to Trevor for the warm welcome and all the help he gave me, at work or outside work, since the beginning of this Ph.D. in the Botany Department. I will always remember his patience and kindness to me at this time. I am also grateful to Vincent for his help and warm welcome during the different periods of time I stayed in London, but especially for all he did for me since my B.Sc. at the University of Lausanne. I wish also to thank Prof. -

Weed Survey of Springvale Station
Weed Survey of Springvale Station Paul Williams Vegetation Management Science August 2016 1 Summary Springvale Station, approximately 50 km to the south-west of Cooktown, covers around 56,000 hectares. The property was purchased by the Queensland Government in May 2016 and is currently managed by the Queensland Department of Environment and Heritage Protection (EHP). A survey of weeds (i.e. invasive exotic plants) observable from the main vehicle tracks on Springvale Station was undertaken by Peter Munt, Keith Smith and Paul Williams on the 4 and 5 August 2016. The survey covered the northern half of the property and included inspections at points on the East, Granite and West Normanby Rivers. It also included surveys of two mine lease areas on the west Normanby River. At each record point, a GPS-derived location was documented and the abundance of all weeds in the surrounding area noted, based on an estimate of their percentage ground cover, grouped into categories (Table 1). Table 1. The abundance categories used for mapping the abundance of each weed (based on the categories used by Weeds of National Significance and the Queensland DPI). Weed Abundance Category Percentage cover of weed Scattered < 1 % cover Low 1 – 9 % cover Moderate 10 – 29 % cover High 30 – 49 % cover Very High ≥ 50 % cover Weed abundance was documented at regular intervals and where weed species composition or abundance changed. A total of 61 weeds were observed (Appendix 1). Nine of the weeds are declared as Category 3 under the new Queensland Biosecurity Act 2014. -

Natural Resource Condition Assessment Horseshoe Bend National Military Park
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Natural Resource Condition Assessment Horseshoe Bend National Military Park Natural Resource Report NPS/SECN/NRR—2015/981 ON THE COVER Photo of the Tallapoosa River, viewed from Horseshoe Bend National Military Park Photo Courtesy of Elle Allen Natural Resource Condition Assessment Horseshoe Bend National Military Park Natural Resource Report NPS/SECN/NRR—2015/981 JoAnn M. Burkholder, Elle H. Allen, Stacie Flood, and Carol A. Kinder Center for Applied Aquatic Ecology North Carolina State University 620 Hutton Street, Suite 104 Raleigh, NC 27606 June 2015 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. The series supports the advancement of science, informed decision-making, and the achievement of the National Park Service mission. The series also provides a forum for presenting more lengthy results that may not be accepted by publications with page limitations. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

Vegetation Community Monitoring at Ocmulgee National Monument, 2011
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 ON THE COVER Duck potato (Sagittaria latifolia) at Ocmulgee National Monument. Photograph by: Sarah C. Heath, SECN Botanist. Vegetation Community Monitoring at Ocmulgee National Monument, 2011 Natural Resource Data Series NPS/SECN/NRDS—2014/702 Sarah Corbett Heath1 Michael W. Byrne2 1USDI National Park Service Southeast Coast Inventory and Monitoring Network Cumberland Island National Seashore 101 Wheeler Street Saint Marys, Georgia 31558 2USDI National Park Service Southeast Coast Inventory and Monitoring Network 135 Phoenix Road Athens, Georgia 30605 September 2014 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Data Series is intended for the timely release of basic data sets and data summaries. Care has been taken to assure accuracy of raw data values, but a thorough analysis and interpretation of the data has not been completed. Consequently, the initial analyses of data in this report are provisional and subject to change. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
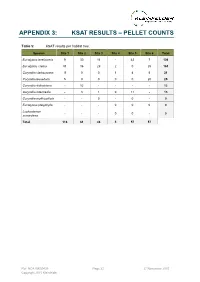
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential