Quantifying Efficiency and Accuracy in the Washington State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Applewood Focused on Apple Varieties
- Advertisement - Applewood focused on apple varieties August 21, 2019 Applewood Fresh Growers LLC is distinguishing itself with planning for the “next best managed varieties,” according to Nick Mascari, Applewood’s president. The Michigan grower-packer-shipper is testing apple varieties in the ground as part of the work toward serving the trade with the best of oncoming special new apple varieties. “We are really looking at making a statement with managed varieties and higher-end apples.” Mascari said that, for competitive reasons, “I can’t be specific” on what is being tested, but “we have five potential new varieties in test blocks.” If those are not ready for production now, “they will be further out. We are on the forefront for new sport varieties, and new strains of old varieties, atop having new managed varieties.” Antonia Mascari was recently promoted to become 1 / 3 the vice president of marketing for Applewood Fresh Growers LLC.With this, Applewood’s sales and marketing arm is “using more data to guide growers and customers” toward the best of the apples that will be coming from Michigan. By tracking data from all growing regions on apple sales for 52 weeks a year, growers can have the best information for planning the timing of new plantings and “we can help guide our customers to have the right assortment at the right times in their stores.” Mascari said USDA statistical data shows “We are the second largest grower-shipper in Michigan. Period. Our growth in volume is due to our team. We are pleased to be the leading sales company in Michigan.” “Our quality and volume will be up this year,” he continued. -

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

APPLE (Fruit Varieties)
E TG/14/9 ORIGINAL: English DATE: 2005-04-06 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * APPLE (Fruit Varieties) UPOV Code: MALUS_DOM (Malus domestica Borkh.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names:* Botanical name English French German Spanish Malus domestica Apple Pommier Apfel Manzano Borkh. The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. Other associated UPOV documents: TG/163/3 Apple Rootstocks TG/192/1 Ornamental Apple * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] i:\orgupov\shared\tg\applefru\tg 14 9 e.doc TG/14/9 Apple, 2005-04-06 - 2 - TABLE OF CONTENTS PAGE 1. SUBJECT OF THESE TEST GUIDELINES..................................................................................................3 2. MATERIAL REQUIRED ...............................................................................................................................3 -

Planting and Aftercare of New Trees
Where to start? • Fruit plants that fit into to small spaces Producing Fruit for the Home – Apple … on dwarfing rootstocks • Most traditional and local garden centers do not identify specific rootstock ….”Dwarf”, “Semi Dwarf” Ron Perry • Eventual tree size within Dwarf and Semi Dwarf is large Professor Tree Spacing Nursery ID Hort. Department Rootstocks Eventual Height Between Trees Between Rows MSU M.27 or P.22 Dwarf 6 5 10 M.9 Dwarf 8 8 12 M.26 Dwarf 16 10 16 M.7 Semi Dwarf 18 14 22 MM.106 or 111 Semi Dwarf 20 16 22 Where to start? Where to start? • Fruit plants that fit into to small spaces – Cherry - Sour • Select desired fruit which will grow in your area. Tree Spacing Rootstocks • Determine how much space you have available. Varieties Eventual Height Between Trees Between Rows Northstar Mahaleb 10 8 12 • Select varieties which are easiest to grow. Montmorency Gi.5 or 6 12 10 12 Montmorency Mahaleb 12 10 14 – Disease or insect resistant varieties to reduce pest Montmorency Mazzard 14 12 16 pressures. Balaton Mahaleb 14 12 16 – Cherry - Sweet – Assess soil / site conditions Tree Spacing • Full sun VS shade or partial Nursery ID • Soil internal drainage Rootstocks Eventual Height Between Trees Between Rows • Weed competition (lawns are too competitive) Gi.5 Dwarf 12 12 16 Gi.6 Dwarf 14 14 16 Mahaleb Semi Dwarf 20 14 16 Mazzard Semi Dwarf 24 16 20 Average Annual Minimum Temperatures Where to start? (USDA Plant Hardiness Zone Map) Most MI fruit sites Zone 5 (-20oF to -10oF) to 6 (-10oF to 0oF) • Fruit plants that fit into to small spaces – Peach, Nectarine, Apricot and Plums – Can generally plant at a spacing of 10 ft X 15 ft* • * If trained to open center or vase shape • Closer spacing, needs to be trained in Chistmas Tree form (Vertical Axe). -

Tree Fruit Culture and Cultivars in North Dakota
H327 (Revised) Tree Fruit Culture and Cultivars in North Dakota Revised by Moving west and north across the state, conditions become less favorable Tom Kalb for growing fruits because of extreme temperatures and reduced rainfall. Horticulturist However, with proper selection and the necessary cultural practices, NDSU Extension Service the home gardener can harvest ample fruit most seasons. Protecting fruit trees from harsh winds in winter and summer cannot Growing your own fruit be overemphasized. City dwellers usually have a more favorable environment for establishing fruit trees than rural residents because the can be fascinating and fun. concentration of buildings and shade trees can greatly reduce wind velocity. Many different kinds of tree On farms, the fruit tree planting is best located within the building site. fruits, including apple, pear, Wind protection may be provided by hills, buildings or shelterbelts. plum and cherry, can be Shelter is necessary from all directions except the east. grown successfully in Fruit trees should be planted at least 40 to 50 feet from farm shelterbelts North Dakota. to prevent breakage from snowdrifts and reduce competition between the fruit trees and shelterbelts for sunlight, soil moisture and nutrients. In town, where backyard space may be limited, locate fruit trees away from large shade trees for similar reasons. Most fruits do well on any fertile garden soil that has good surface drainage. July 2016 1 • H327 Tree Fruit Culture and Cultivars in North Dakota How to Select Cultivars Well-branched trees can be developed Gardeners in northern areas will by the homeowner even if only a Fruit Tree benefit from choosing the most healthy, unbranched trees between 5 and 6 feet tall are available. -
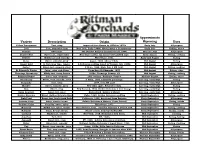
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in Rosbreed
Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in RosBREED Jim Luby, Breeding Team Leader Outline of Presentation RosBREED Demonstration Breeding Programs Standardized Phenotyping Protocols Reference Germplasm Sets SNP Detection Panels Crop Reference Set Breeding Pedigree Set RosBREED Demonstration Breeding Programs Clemson U WSU Texas A&M UC Davis U Minn U Arkansas Rosaceae Cornell U WSU MSU MSU Phenotyping Affiliates USDA-ARS Driscolls Corvallis Univ of Florida UNH Standardized Phenotyping Protocols Traits and Standardized Phenotyping Protocols • Identify critical fruit quality traits and other important traits • Develop standardized phenotyping protocols to enable data pooling across locations/institutions • Protocols available at www.RosBREED.org Apple Standardized Phenotyping Firmness, Crispness – Instrumental, Sensory Sweetness, Acidity – Intstrumental, Sensory Color, Appearance, Juiciness, Aroma – Sensory At harvest Cracking, Russet, Sunburn Storage 10w+7d Storage 20w+7d Maturity Fruit size 5 fruit (reps) per evaluation Postharvest disorders Harvest date, Crop, Dropping RosBREED Apple Phenotyping Locations Wenatchee, WA St Paul, MN Geneva, NY • One location for all evaluations would reduce variation among instruments and evaluators • Local evaluations more sustainable and relevant for future efforts at each institution • Conduct standardized phenotyping of Germplasm Sets at respective sites over multiple (2-3) seasons • Collate data in PBA format, conduct quality control, archive Reference -

BIX Minnesota Grown Apples Local 2018 Local Availability Apples
BIX BIX Minnesota Grown Apples Local 2018 Local Availability Apples *Apple varieties & availability vary per orchard and growing conditions. These are approximate starting times for your favorite varieties. Paula Reds Honeycrisp An early season favorite, this apple is great for A great mid-season apple with a crispy and juicy cooking and eating, as it is not too tart and not texture makes Honeycrisp a customer favorite! to sweet. Available week of 9/17 Available week of 8/27 BIX Item #1000 #1021 BIX Item #1050 SweeTango Zestar Great for salads, this variety is sweet, juicy, & crisp. One of the newest varieties from the U of M, *Pepin Heights Orchard Exclusive variety* this early season apple has a crisp, juicy texture and a zesty flavor with a hint of brown sugar. Available week of 9/3 Available week of 9/3 BIX Item #1385 BIX Item #1320 Haralson Still Midwest’s favorite baking apple, it will be Riverbelle perfect for your Grandma’s Apple Pie recipe! This variety makes for a good all purpose apple, with sweet flavor and great crispness. Available week of 10/8 *Very limited availability, but large in size!* BIX Item #1015 #1200 Available week of 9/10 Pazazz BIX Item #1376 Perfect balance of brix and starch; explosive crunch; very sweet with McIntosh a subtle tart flavor, similar to a honeycrisp. This variety is a smaller apple with red and green tender flesh. This apples is great for pies, Available week of 10/29 cider and applesauce, as well as school lunches. BIX Item #1311 Available week of 9/14 Apple Cider BIX Item #1310 #1500 Apple Cider Cortland Fresh pressed cider to capture the flavors This variety is versatile and is a perfect and aromas of the apple harvest season. -

Apples: Organic Production Guide
A project of the National Center for Appropriate Technology 1-800-346-9140 • www.attra.ncat.org Apples: Organic Production Guide By Tammy Hinman This publication provides information on organic apple production from recent research and producer and Guy Ames, NCAT experience. Many aspects of apple production are the same whether the grower uses low-spray, organic, Agriculture Specialists or conventional management. Accordingly, this publication focuses on the aspects that differ from Published nonorganic practices—primarily pest and disease control, marketing, and economics. (Information on March 2011 organic weed control and fertility management in orchards is presented in a separate ATTRA publica- © NCAT tion, Tree Fruits: Organic Production Overview.) This publication introduces the major apple insect pests IP020 and diseases and the most effective organic management methods. It also includes farmer profiles of working orchards and a section dealing with economic and marketing considerations. There is an exten- sive list of resources for information and supplies and an appendix on disease-resistant apple varieties. Contents Introduction ......................1 Geographical Factors Affecting Disease and Pest Management ...........3 Insect and Mite Pests .....3 Insect IPM in Apples - Kaolin Clay ........6 Diseases ........................... 14 Mammal and Bird Pests .........................20 Thinning ..........................20 Weed and Orchard Floor Management ......20 Economics and Marketing ........................22 Conclusion -

Cosmic Crisp's Growth Is out of This World
- Advertisement - Cosmic Crisp's growth is out of this world 1 / 2 April 26, 2021 After a record-breaking sophomore year that hasn't finished, the Cosmic Crisp apple’s growth trajectory indicates it will launch to the top of the apple chart in no time. Nielsen data show monumental growth, reaching No. 11 in sales dollars in March, and No. 14 over the 52 weeks ending March 27. If retailers do not have a Cosmic Crisp apple program in place now, they are missing out on very important sales and customer expectations. “Cosmic Crisp volume is planned to more than double this fall, and double again for the 2022 harvest. This volume will rival current core variety volume, displacing mature varieties that are being replaced with higher color strains and higher flavor varieties,” said Catherine Gipe-Stewart, communications manager. “Cosmic Crisp is rising so quickly, it has potential to become a top-five variety at this time next year.” Nielsen data show a 595 percent increase in dollars and a 720 percent in volume over the four weeks ending March 27. In March alone, Cosmic Crisp earned the 11th spot with $4.3 million in sales and 1.8 million pounds, at an average price of $2.52 per pound, right in line with Honeycrisp at $2.55 per pound. Looking at a yearly perspective (last 52 weeks), Cosmic Crisp has earned $20 million in sales, with 7.7 million pounds. “Historically, apples like Cosmic Crisp take root in the Pacific region, and spread eastward like wildfire,” said Gipe-Stewart. -

Open KON Dissertation FINAL.Pdf
The Pennsylvania State University The Graduate School College of Agricultural Sciences EVALUATION OF CHEMICAL AND THERMAL BLOSSOM THINNING STRATEGIES FOR APPLE A Dissertation in Horticulture by Thomas M. Kon 2016 Thomas M. Kon Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2016 The dissertation of Thomas M. Kon was reviewed and approved* by the following: James R. Schupp Professor of Pomology Dissertation Advisor Chair of Committee Robert M. Crassweller Professor of Horticulture Paul Heinemann Professor and Head of Agricultural and Biological Engineering Richard Marini Professor of Horticulture Erin L. Connolly Professor of Plant Science Head of the Department of Plant Science *Signatures are on file in the Graduate School iii ABSTRACT Since 1989, approximately 150 compounds and multiple mechanical devices were evaluated as apple blossom thinners. Despite these efforts, blossom thinner adoption has been limited to a few apple producing regions or states. The purpose of this work was to: 1) compare the efficacy of promising chemical blossom thinners using a predictive model as a timing aid, and 2) evaluate the potential of short-duration thermal treatments as a blossom thinning strategy. Using a predictive model as a timing aid, the efficacy of several promising chemical blossom thinners was evaluated at Penn State’s Fruit Research and Extension Center in Biglerville, PA. Blossom thinner effects on pollen tube growth, fruit set, and yield responses were evaluated. Calcium polysulfide and ammonium thiosulfate inhibited pollen tube growth in vivo and reduced initial fruit set. Endothal was a potent thinner, but was ineffective in reducing pollen tube growth and caused excessive leaf injury. -

Systematically Integrating DNA Information Into Breeding: the MAB
Systematically integrating DNA information into breeding: The MAB Pipeline, case studies in apple and cherry Amy Iezzoni January 31, 2013 Cornell MSU Susan Brown Amy Iezzoni (PD) Kenong Xu Jim Hancock Dechun Wang Clemson Cholani Weebadde Ksenija Gasic Gregory Reighard Univ. of Arkansas John Clark WSU Texas A&M USDA-ARS Dave Byrne Cameron Peace Nahla Bassil Dorrie Main Univ. of Minnesota Gennaro Fazio Univ. of CA-Davis Kate Evans Chad Finn Karina Gallardo Jim Luby Tom Gradziel Vicki McCracken Chengyan Yue Plant Research Intl, Carlos Crisosto Nnadozie Oraguzie Netherlands Oregon State Univ. Eric van de Weg Univ. of New Hamp. Alexandra Stone Marco Bink Tom Davis Outline of Presentation The MAB Pipeline Apple skin color Cherry flesh color The MAB Pipeline “Jewels in the Genome” - discovering, polishing, applying QTL discovery MAB Pipelining Breeding (looks promising...) (polishing...) (assembling into masterpieces) Socio-Economics Surveys (example for apple) Washington Michigan Market Breeders Producers Producers Intermediaries Fruit flavor 43 41 23 Fruit crispness 15 23 10 Exterior color 26 Fruit firmness 6 7 5 Shelf life at retail 7 7 3 Sweetness/soluble solids 6 7 3 Sugar/acid balance 9 7 External appearance 13 No storage disorders 7 4 Disease resistance 2 5 Storage life 5 Other fruit quality…2 3 Size 3 Juiciness 2 Tartness Shape Phytonutrient Aroma % of respondents020406080100 Reference Germplasm McIntosh Melba LivelRasp Jolana Williams F_Spartan Spartan PRI14-126 Starr OR38T610 F_Williams NJ53 PRI14-226 Minnesota Delicious KidsOrRed