Diversity and Evolution of the Middle American Cichlid Fishes (Teleostei: Cichlidae) with Revised Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non-Native Freshwater Fish Management in Biosphere Reserves
ISSN 1989‐8649 Manag. Biolog. Invasions, 2010, 1 Abstract Non‐Native freshwater fish management in Biosphere Reserves The consideration of non‐native freshwater fish species in the Andrea PINO‐DEL‐CARPIO*, Rafael MIRANDA & Jordi PUIG management plans of 18 Biosphere Reserves is evaluated. Additionally, impacts caused by introduced freshwater fish species are described. Introduction, Hypotheses and Reserves was created in 1974 and it Some measures to alleviate the Problems for Management is defined in the Statutory ecological effects of fish species Framework of the World network as introductions are proposed, while Biodiversity, the variety of life on “areas of terrestrial and coastal/ paying attention to local development Earth, is disappearing at an marine ecosystems, or a as well. The introduction of non‐native combination thereof, which are species may have negative conse‐ increasing and unprecedented rate quences for the ecosystems. The (Rockström et al. 2009). This internationally recognized within analysis of the management plans of situation contradicts the 2002 the framework of UNESCO the Reserves confirms that non‐native objective to achieve a significant Programme on Man and the freshwater fish species sometimes are reduction of the current rate of Biosphere (MaB)” (UNESCO 1996). not considered in the action plans of biodiversity loss by 2010 To what extent Biosphere Reserves the area. Biosphere Reserve’s (Convention on Biological Diversity fulfil the aim of biodiversity management plans should consider the 2002). One of the multiple initiatives conservation? In order to make a presence of alien species, with the aim adopted to promote biodiversity first inquiry in this issue, some to preserve biodiversity. -

Pandolfi, Matías. 2005
Tesis Doctoral Caracterización de las variantes de GnRH y las gonadotrofinas en Cichlasoma dimerus (Teleostei, Perciformes) y su relación con la diferenciación sexual Pandolfi, Matías 2005 Este documento forma parte de la colección de tesis doctorales y de maestría de la Biblioteca Central Dr. Luis Federico Leloir, disponible en digital.bl.fcen.uba.ar. Su utilización debe ser acompañada por la cita bibliográfica con reconocimiento de la fuente. This document is part of the doctoral theses collection of the Central Library Dr. Luis Federico Leloir, available in digital.bl.fcen.uba.ar. It should be used accompanied by the corresponding citation acknowledging the source. Cita tipo APA: Pandolfi, Matías. (2005). Caracterización de las variantes de GnRH y las gonadotrofinas en Cichlasoma dimerus (Teleostei, Perciformes) y su relación con la diferenciación sexual. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Cita tipo Chicago: Pandolfi, Matías. "Caracterización de las variantes de GnRH y las gonadotrofinas en Cichlasoma dimerus (Teleostei, Perciformes) y su relación con la diferenciación sexual". Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. 2005. Dirección: Biblioteca Central Dr. Luis F. Leloir, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. Contacto: [email protected] Intendente Güiraldes 2160 - C1428EGA - Tel. (++54 +11) 4789-9293 Universidad de Buenos Aires, Argentina Facultad de Ciencias Exactas y Naturales Departamento de Biodiversidad y Biología Experimental “Caracterización de las variantes de GnRH y las gonadotrofinas en Cichlasoma dimerus (Teleostei, Perciformes) y su relación con la diferenciación sexual” Trabajo de Tesis presentado para optar por el título de Doctor de la Universidad de Buenos Aires Autor: Lic. -

Evaluating Longitudinal Changes in Behavior and 11-Ketotestosterone in Parental Convict Cichlids (Amatitlania Siquia)
Georgia State University ScholarWorks @ Georgia State University Biology Theses Department of Biology 5-10-2019 PAIR BOND DYNAMICS: EVALUATING LONGITUDINAL CHANGES IN BEHAVIOR AND 11-KETOTESTOSTERONE IN PARENTAL CONVICT CICHLIDS (AMATITLANIA SIQUIA) Celine Richards Follow this and additional works at: https://scholarworks.gsu.edu/biology_theses Recommended Citation Richards, Celine, "PAIR BOND DYNAMICS: EVALUATING LONGITUDINAL CHANGES IN BEHAVIOR AND 11-KETOTESTOSTERONE IN PARENTAL CONVICT CICHLIDS (AMATITLANIA SIQUIA)." Thesis, Georgia State University, 2019. https://scholarworks.gsu.edu/biology_theses/92 This Thesis is brought to you for free and open access by the Department of Biology at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Biology Theses by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. PAIR BOND DYNAMICS: EVALUATING LONGITUDINAL CHANGES IN BEHAVIOR AND 11-KETOTESTOSTERONE IN PARENTAL CONVICT CICHLIDS (AMATITLANIA SIQUIA) By CELINE RICHARDS Under the Direction of Edmund Rodgers, PhD ABSTRACT Bi-parental care and pair bonding often coincide in nature. The reproductive success of the organisms that apply this strategy is dependent upon defensive behaviors and territorial aggression. Some of these organisms also display affiliative behavior within the pair pond during the time of parental care. The behavioral dynamics that occur over the course of the pair bond and their relationship to the reproductive success of the organism is not well understood. Convict cichlids (Amatitlania siquia) form pair bonds during the breeding season and provide bi- parental care; their behavioral repertoire is ideal for studying pair bonding. The androgen profile of organisms that provide parental care through aggressive means is also not fully understood. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Temporal Diversification of Mesoamerican Cichlid Fishes Across
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 31 (2004) 754–764 www.elsevier.com/locate/ympev Temporal diversification of Mesoamerican cichlid fishes across a major biogeographic boundary C. Darrin Hulsey,a,* Francisco J. Garcıa de Leon, b Yara Sanchez Johnson,b Dean A. Hendrickson,c and Thomas J. Neara,1 a Center for Population Biology, Department of Evolution and Ecology, University of California-Davis, Davis, CA 95616, USA b Laboratorio de Biologıa Integrativa, Instituto Tecnologico de Cuidad Victoria (ITCV), Mexico c Section of Integrative Biology, University of Texas-Austin, Austin, TX 78712, USA Received 18 June 2003; revised 26 August 2003 Abstract The Mexican Neovolcanic Plateau sharply divides the vertebrate fauna of Mesoamerica where the climate of both the neotropics and temperate North America gradually blend. Only a few vertebrate groups such as the Heroine cichlids, distributed from South America to the Rio Grande in North America, are found both north and south of the Neovolcanic Plateau. To better understand the geography and temporal diversification of cichlids at this geologic boundary, we used mitochondrial DNA sequences of the cy- tochrome b (cyt b) gene to reconstruct the relationships of 52 of the approximately 80 species of Heroine cichlids in Mesoamerica. Our analysis suggests several cichlids in South America should be considered as part of the Mesoamerican Heroine clade because they and the cichlids north of the Isthmus of Panama are clearly supported as monophyletic with respect to all other Neotropical cichlids. We also recovered a group containing species in Paratheraps + Paraneetroplus + Vieja as the sister clade to Herichthys. Herichthys is the only cichlid clade north of the Mexican Plateau and it is monophyletic. -

They're Not Just Convicts Anymore
2008 FAAS Publication Awards. Please follow reprint instructions at http://www.faas.info/2008_publication_awards_winners.html#reprintpolicy They’re Not Just Convicts Anymore By Daniel Spielman All Photos by Daniel Spielman Introduction Convicts get no respect, with many a cichlidophile turning up his or her nose at the sight of a group on Convicts in a tank or a bag of fry in an auction. It’s time for that to change. Easy to breed and exhibiting wonderful parental care, Convict cichlids (Cryptoheros nigrofasciatus) have long been staples in the hobby. Indeed, for many new fishkeepers, the satisfaction of watching a pair of Convict parents herd a group of fry around a tank sparks the initial desire to keep other mem bers of the cichlid family. Indeed, my fish cichlids were Convicts, and watch ing them care for their fry definitely got me hooked. The contrast with trying to keep guppies or swordtails from eating their own offspring is striking (how did eating one’s own young ever evolve in the first place?). However, the very fact that Convicts spawn so readily in the aquarium causes many hobbyists to quickly lose interest in the species. Although Convicts occasionally appear on experienced hobbyists’ lists of most favorite cichlid, the problem is they are just too common and too easy to breed. Typical Convict spawning jokes involve two fish and a wet paper towel, and one often feels fortunate to break the $1 barrier at the auction to avoid the indignity of having to bring one’s fish back home again at the end of the monthly club meeting. -

Description of Two New Species of the Midas Cichlid Complex (Teleostei: Cichlidae) from Lake Apoyo, Nicaragua
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 123(2):159–173. 2010. Description of two new species of the Midas cichlid complex (Teleostei: Cichlidae) from Lake Apoyo, Nicaragua Matthias F. Geiger, Jeffrey K. McCrary, and Jay R. Stauffer, Jr.* (MFG) Bavarian State Collection of Zoology (ZSM), Department of Ichthyology, Mu¨nchhausenstr. 21, 81247 Munich, Germany; (JKM) Fundacio´n Nicaragu¨ense Pro-desarrollo Comunitario Integral (FUNDECI/GAIA), Estacio´n Biolo´gica, Laguna de Apoyo Nature Reserve, Nicaragua; (JRS) School of Forest Resources, 432 Forest Resources Building, The Pennsylvania State University, University Park, Pennsylvania 16802, U.S.A., e-mail: [email protected] Abstract.—Two species belonging to the Amphilophus citrinellus (Gu¨nther, 1864) species complex endemic to Lake Apoyo, Nicaragua are described. Both species exhibit unique phenotypic characters that have not been found in other members of the species complex. Furthermore, they breed assortatively in Lake Apoyo and can readily be distinguished in the field from all other described species found in that lake. Including the two herein described species, six species that form a monophyletic species assemblage within the Midas cichlid complex inhabit Lake Apoyo, Nicaragua. Midas cichlids are monogamous sub- allowed the description of three endemic strate spawners that form pairs only species from Lake Xiloa´ in 2002 and three during breeding season, when they ag- endemic species from Lake Apoyo in gressively defend their territory and fry. 2008 (Stauffer & McKaye 2002, -

Rapid Evolution and Selection Inferred from the Transcriptomes of Sympatric Crater Lake Cichlid Fishes
Rapid evolution and selection inferred from the transcriptomes of sympatric crater lake cichlid fishes K. R. ELMER, S. FAN, H. M. GUNTER, J. C. JONES, S. BOEKHOFF, S. KURAKU and A. MEYER Lehrstuhl fUr Zoologie und Evolutionsbiologie, Department of Biology, University of Konstanz, Universitiitstrasse 10, 78457 Konstanz, Germany Abstract Crater lakes provide a natural laboratory to study speciation of cichlid fishes by ecological divergence. Up to now, there has been a dearth of transcriptomic and genomic information that would aid in understanding the molecular basis of the phenotypic differentiation between young species. We used next-generation sequencing (Roche 454 massively parallel pyrosequencing) to characterize the diversity of expressed sequence tags between ecologically divergent, endemic and sympatric species of cichlid fishes from crater lake Apoyo, Nicaragua: benthic Amphilophus astorquii and limnetic Amphilophus zaliosus. We obtained 24174 A. astorquii and 21382 A. zaliosus high quality expressed sequence tag contigs, of which 13 106 pairs are orthologous between species. Based on the ratio of non synonymous to synonymous substitutions, we identified six sequences exhibiting signals of strong diversifying selection (KalKs > 1). These included genes involved in biosynthesis, metabolic processes and development. This transcriptome sequence variation may be reflective of natural selection acting on the genomes of these young, sympatric sister species. Based on Ks ratios and p-distances between 3'-untranslated regions (UTRs) calibrated to previously published species divergence times, we estimated a neutral transcriptome-wide substitutional mutation rate of ~1.25 x 10-6 per site per year. We conclude that next-generation sequencing technologies allow us to infer natural selection acting to diversify the genomes of young species, such as crater lake cichlids, with much greater scope than previously possible. -

What Is a Species? an Endless Debate
GENERAL ARTICLE What is a Species? An Endless Debate Uttam Saikia, Narayan Sharma and Abhijit Das Everybody is familiar with the ubiquitous term ‘species’.But what is a species? Undeniably, this is one of the most complex dilemmas in the history of biology. There is no other concept in biology as elementary yet controversial as the concept of species. In practice, a species is regarded as the fundamental unit of comparison in all biological disciplines like systemat- ics, evolution, ecology, ethology, physiology, and genetics. However, little agreement exists in the scientific community regarding the nature of species, whether it is a real entity or (left) Uttam Saikia is a cultural artifact, its biological significance or how to delin- working at High Altitude eateaspecies. Thisarticleisareview oftheseeminglyendless Zoology Field Station, debate on the species concept and its implications. Zoological Survey of India, Solan, Himachal Pradesh. The Species Concept: Historical Perspective (right) Narayan Sharma is Since olden times, naturalists have felt the need for a unit by a research scholar in National Institute of which diversity of life form on Earth can be described and Advanced Studies, IISc measured. Early naturalists and philosophers like Pluto and Campus, Bangalore. His Aristotle initiated the process of classifying biological objects area of research is primate based on typological essentialism i.e., every species (species is ecology and behaviour. the Latin word for kind) should conform to certain predetermined (center) Abhijit Das is a ideal “type”. Anything not similar to a particular type is consid- research scholar in Utkal ered a different species. This is known as typological species University, Orissa. -
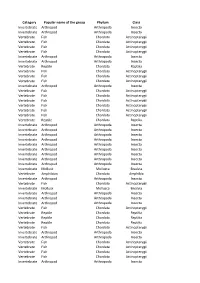
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Cytogenetics of Gymnogeophagus Setequedas (Cichlidae: Geophaginae), with Comments on Its Geographical Distribution
Neotropical Ichthyology, 15(2): e160035, 2017 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20160035 Published online: 26 June 2017 (ISSN 1982-0224) Copyright © 2017 Sociedade Brasileira de Ictiologia Printed: 30 June 2017 (ISSN 1679-6225) Cytogenetics of Gymnogeophagus setequedas (Cichlidae: Geophaginae), with comments on its geographical distribution Leonardo M. Paiz1, Lucas Baumgärtner2, Weferson J. da Graça1,3, Vladimir P. Margarido1,2 and Carla S. Pavanelli1,3 We provide cytogenetic data for the threatened species Gymnogeophagus setequedas, and the first record of that species collected in the Iguaçu River, within the Iguaçu National Park’s area of environmental preservation, which is an unexpected occurrence for that species. We verified a diploid number of 2n = 48 chromosomes (4sm + 24st + 20a) and the presence of heterochromatin in centromeric and pericentromeric regions, which are conserved characters in the Geophagini. The multiple nucleolar organizer regions observed in G. setequedas are considered to be apomorphic characters in the Geophagini, whereas the simple 5S rDNA cistrons located interstitially on the long arm of subtelocentric chromosomes represent a plesiomorphic character. Because G. setequedas is a threatened species that occurs in lotic waters, we recommend the maintenance of undammed environments within its known area of distribution. Keywords: Chromosomes, Conservation, Iguaçu River, Karyotype, Paraná River. Fornecemos dados citogenéticos para a espécie ameaçada Gymnogeophagus setequedas, e o primeiro registro da espécie coletado no rio Iguaçu, na área de preservação ambiental do Parque Nacional do Iguaçu, a qual é uma área de ocorrência inesperada para esta espécie. Verificamos em G. setequedas 2n = 48 cromossomos (4sm + 24st + 20a) e heterocromatina presente nas regiões centroméricas e pericentroméricas, as quais indicam caracteres conservados em Geophagini. -

Thesis Ebi Antony George
Individual and social mechanisms regulating the dance activity within honey bee forager groups A Thesis Submitted to the Tata Institute of Fundamental Research, Mumbai for the degree of Doctor of Philosophy in Biology by Ebi Antony George National Centre for Biological Sciences, Bangalore Tata Institute of Fundamental Research, Mumbai October 2019 Declaration Date: 11 October 2019 iii Certificate v Publications George EA, Brockmann A. 2019 Social modulation of individual differences in dance communication in honey bees. Behav. Ecol. Sociobiol. 73, 41. (doi:10.1007/s00265-019- 2649-0) Content from the above publication has been incorporated in the thesis with permission from the publisher (License no: 4570571255954). George EA, Bröger A-K, Thamm M, Brockmann A, Scheiner R. 2019 Inter-individual variation in honey bee dance intensity correlates with expression of the foraging gene. Genes, Brain and Behavior. 1– 11. (doi:10.1111/gbb.12592) Content from the above publication has been incorporated in the thesis with permission from the publisher (License no: 4675421240663). vii Acknowledgements Charles Darwin rightly said that “it is the long history of humankind (and animal kind, too) that those who learned to collaborate and improvise most effectively have prevailed”. These words highlight an interesting parallel between the paradigm I studied (the foraging activity of the honey bee colony) and my own experience during my PhD at the National Centre for Biological Sciences, Bangalore. In both cases, any successful endeavour depended on the interaction between a diverse group of individuals, each supporting the other. Even though it is my name on the first page of this thesis, I am part of a multitude of people who have all contributed academically and otherwise to this document.