Peach Fruit Fly, Bactrocera Zonata, Host List the Berries, Fruit, Nuts and Vegetables of the Listed Plant Species Are Now Considered Host Articles for B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New World Guava Fruit Fly, Anastrepha Striata, Host List the Berries, Fruit, Nuts and Vegetables of the Listed Plant Species Are Now Considered Host Articles for A
November 2018 New World Guava Fruit Fly, Anastrepha striata, Host List The berries, fruit, nuts and vegetables of the listed plant species are now considered host articles for A. striata. Unless proven otherwise, all cultivars, varieties, and hybrids of the plant species listed herein are considered suitable hosts of A. striata. Scientific Name Common Name Acca sellowiana (O. Berg) Burret Pineapple-guava, feijoa Anacardium occidentale L. Cashew, cajuil Annona cherimola Mill. Cherimoya, custard-apple Annona muricata L. Soursop, araticum-grande Averrhoa carambola L. Carambola, starfruit Bellucia dichotoma Cogn. N/A Bellucia grossularioides (L.) Triana N/A Byrsonima crassifolia (L.) Kunth Craboo, golden-spoon Calycolpus moritzianus (O. Berg) Burret N/A Campomanesia lineatifolia Ruiz & P av. Guabiroba, guayaba de leche Carica papaya L. Papaya, pawpaw 1 Citrus xsinensis (L.) Osbeck Sweet orange, blood orange Citrus xtangeloJ. W. Ingram & H. E. Moore Tangelo, uglifruit Coffea arabica L. Arabica coffee, Arabian coffee Couma utilis (Mart.) Mull. Arg. Sorva, sorva pequena Diospyros digyna Jacq. Black persimmon, black sapote Eriobotrya japonica (Thunb). Lindl. Loquat, Japanese-medlar Eugenia ligustrina (Sw.) Willd. Birchberry, privet stopper Eugenia luschnathiana (O. Berg) Klotzsch ex B. D. Jacks N/A Eugenia stipitata McVaugh Araca-boi, araza Eugenia uniflora L. Brazil-cherry, Surinam-cherry Inga edulis Mart. Ice-cream-bean, inga-cipo Inga feuilleei DC. Pacae, pacay Inga velutina Wiild. N/A Malpighia glabra L. Escobillo Mangifera indica L. Common mango, Indian mango Manilkara zapota (L.) P. Royen Sapote, naseberry, sapodilla Oenocarpus bacaba Mart. Bacaba palm Common passionfruit, purple Passiflora edulis Sims granadilla Persea americana Mill. Avocado, abacate 2 Pouteria caimito (Ruiz & Pav.) Radlk. -

Native Trees of Georgia
1 NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION Eleventh Printing - 2001 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

Nutrition Facts N *Learn About Fiber on Page 2
Health and Learning Success Go Hand-In-Hand California’s geography offers a bounty of fresh produce and recreational areas. From stone fruits and salad greens to state and local parks, there is no shortage of healthy foods to eat and outdoor activities to do in California. Studies show that healthy eating and physical activity are correlated with improved academic achievement. Use Harvest of the Month to allow students to experience California-grown fruit and vegetables with their senses. Teach students to live a healthy, active lifestyle and support academic content standards to link the classroom, cafeteria, home, and community. Exploring California Peaches: Taste Testing Network for a Healthy California What You Will Need (per group of 8 students): n Four ripe peaches and four ripe nectarines (two each of yellow and white varieties)* n Paring knife and cutting board n Paper towels *Choose peaches and nectarines that are fragrant and firm to slightly soft when pressed. Optional: Sample other stone fruits (cherries, plums, apricots, etc.) with peaches. Activity: n Distribute yellow peaches and nectarines to each student group. n Observe the look, feel, and smell of each; record observations. n Cut open the second yellow fruit; observe the taste and sound and record observations. n Repeat with white peaches and nectarines. n Discuss similarities and differences among the four varieties. n Record students’ favorite variety; share results with school nutrition staff. For more ideas, reference: Nutrition to Grow On, CDE, 2001. Cooking in Class: Reasons to Eat Peaches Peach Smoothies A ½ cup of sliced peaches (about half of Makes 35 tastes at ¼ cup each a medium peach) provides: Ingredients: n A source of vitamin A and vitamin C. -

The Influence of Invasive Alien Plant Control on the Foraging Habitat Quality of the Mauritian Flying Fox (Pteropus Niger)
Master’s Thesis 2017 60 ECTS Department of Ecology and Natural Resource Management The influence of invasive alien plant control on the foraging habitat quality of the Mauritian flying fox (Pteropus niger) Gabriella Krivek Tropical Ecology and Natural Resource Management ACKNOWLEDMENTS First, I would like to express my gratitude to my supervisors Torbjørn Haugaasen from INA and Vincent Florens from the University of Mauritius, who made this project possible and helped me through my whole study and during the write-up. I also want to thank to NMBU and INA for the financial support for my field work in Mauritius. I am super thankful to Maák Isti for helping with the statistical issues and beside the useful ideas and critical comments, sending me positive thoughts on hopeless days and making me believe in myself. Special thanks to Cláudia Baider from The Mauritius Herbarium, who always gave me useful advice on my drafts and also helped me in my everyday life issues. I would like to thank Prishnee for field assistance and her genuine support in my personal life as well. I am also grateful to Mr. Owen Griffiths for his permission to conduct my study on his property and to Ravi, who generously provided me transportation to the field station, whenever I needed. Super big thanks to Rikard for printing out and submitting my thesis. I am also thankful to my family for their support during my studies, fieldwork and writing, and also for supporting the beginning of a new chapter in Mauritius. Finally, a special mention goes to my love, who has been motivating me every single day not to give up on my dreams. -

Queensland Brain Institute 2017 Annual Report Director’S Message
Queensland Brain Institute 2017 Annual Report Director’s message 2017 was another year of strong achievement in our research output, as reflected in the number and quality of our peer-reviewed publications in leading scientific journals, and our grant success in both the NHMRC Project Grant and the ARC Discovery Project schemes. It was particularly pleasing that many of our younger recruits obtained grant funding. QBI’s clinical links were strengthened through a joint appointment with Mater Neuroscience; Professor Peter Nestor, a clinical neurologist from the German Center for Neurodegenerative Diseases, started in October. Refurbishment of the Ritchie building occurred during much of the year, and a number of Faculty have moved across into the new space; the breakthrough from the Clem Jones Centre for Ageing Dementia Research (CJCADR) into the Ritchie building on Level 3 is also complete, and seamlessly links the two buildings. This space forms a visible clinical interface for QBI, and will include a Memory Clinic for testing patients with neurodegenerative diseases. In August, QBI hosted the first official visit of the newly appointed International Research Review Board. Half of the Faculty were reviewed, and the research focus, current and future, of the Institute was reviewed. The remaining half are also scheduled for review in December 2018. The initial Report was very supportive, and noted that the breadth of neuroscience research was impressive, and that QBI is a world-leading Institute that enjoys high international visibility. Recommendations outlined in the Report will be examined in detail over the coming year. Professor Pankaj Sah Director, Queensland Brain Institute Queensland Brain Institute Annual Report 2017 1 Research highlights There were several major research discoveries in 2017, as well as the progression of a number of research outcomes towards commercialisation. -

Plant Biometrics of Malay, Rose and Water Apple Guilherme Nacata1 & Renata Aparecida De Andrade2
ISSN 0100-2945 DOI: http://dx.doi.org /10.1590/0100-29452018131 Botanic and Physiology Plant biometrics of malay, rose and water apple Guilherme Nacata1 & Renata Aparecida de Andrade2 Abstract - Due to the lack of studies comparing Syzygium malaccensis(L) Meer & Perry, Syzygium aqueum Burm.f. and Syzygium jambos (L) Alston Syzygium in relation to the botanical genus Sygygium regarding the physical characteristics, the present research was conducted, aiming at biometrically characterizing each of the three species, as well as comparing them. The fruits were collected from the São Paulo State University (Unesp), School of Agricultural and Veterinarian Sciences, Jaboticabal. Syzygium plants were used for the evaluation of: fruit and seed masses (g); width and length of leaves (cm) and of seeds (cm); percentage of fruit pulp; length of leaf petiole (cm); and leaf area (cm2). Fifty fruits, leaves and seeds of each species were used, with 5 replicates of 10 samples each and the data were submitted to descriptive statistical analysis and organized into frequency tables. For comparative purposes among the species, a blocks randomized design was adopted, being analyzed by the Tukey Test at 5% of probability. The malay and water apple present fruits with high yield of pulp. The fruit of the malay apple are light, with narrow diameter, medium length, broad seeds, narrow and small leaves, with small leaf area. The rose apple fruit present medium mass, width and length, with broad seeds, narrow leaves, medium length and small leaf area. The water apple fruit are light, broad, long, without seeds, with broad leaves, long and having medium leaf area. -

Bactrocera Tryoni: Host Crops and Other Plants
Bactrocera tryoni: Host Crops and Other Plants This list has been compiled from several online sources: http://www.agric.wa.gov.au/objtwr/imported_assets/content/pw/ins/pp/hort/fs04300.pdf http://www.pestfreearea.com.au/host-list-of-banned-poduce.html http://www.spc.int/Pacifly http://www.bugsforbugs.com.au/pdf/cms/QldFruitFlyHostList.pdf abiu (Pouteria caimito) Indian plum (Flacourtia jangomas) acerola (Malpighia emarginata) jaboticaba (Plinia caluiflora) apple (Malus) jackfruit (Artocarpus heterophyllus) apricot (Prunus armeniaca) jew plum (golden apple, Spondias cytherea) Australian desert lime (Eremocitrus glauca) ju jube (Ziziphus mauritiana) avocado (Persea americana) kangaroo apple (Solanum laciniatum) babaco (Carica pentagoona) kei apple (Dovyalis caffra) banana (Musa sp.) kiwi fruit (Actinidia sp.) black mulberry (Morus nigra) kumquat (Fortunella japonica) black sapote (Diospyros digyna) lemon (Citrus limon) blackberry (Rubus fruticosus) lime (Citrus sp.) blueberry (Vaccinium sp.) loganberry (Rubus × loganobaccus) Brazil cherry (pitanga, Surinam cherry, or cayenne cherry, longan(Dimocarpus longan) Eugenia uniflora) loquat (Eriobotrya japonica) breadfruit (Artocarpus altilis) lychee (Litchi chinensis) caimito (star apple, Chrysophyllum cainito) mandarin (Citrus reticulate) California berry (Rubus ursinus) mango (Mangifera indica) Cape gooseberry (Physalis peruviana) mangosteen (Garcinia mangostana) capsicum (Capsicum sp.) medlar (Mespilus germanica) carambola (star fruit, Averrhoa carambola) miracle fruit (Synsepalum dulcificum) -
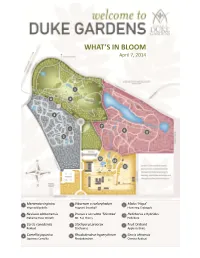
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Peony, Powdery Mildew (Erysiphe Polygoni)
Problem: Peony, Powdery Mildew (Erysiphe polygoni) Host Plants: Peony Description: Powdery mildew starts as individual spots that resemble snowflakes but rapidly coalesce to cover the entire leaf so that a plant look like it was dusted with flour. Though common on other plants such as lilac and bee balm, it has been relatively rare on peony until the last few years. Recommendations: Poor air movement and shade make the disease more likely. Growing peonies in full sun with good air movement will help minimize the disease. Fungicides can be effective if applied before infection has occurred. Therefore, heavily infected plants should not be treated as the treatment will be ineffective. Fortunately, the disease should cause no lasting damage to the plant. Remove and discard (or compost) infected plant material at the end of the season. Look for individual spots to appear the following spring and then apply a recommended fungicide before the disease has spread. Suggested fungicides include myclobutanil (Eagle, Spectracide Immunox, Monterey Fungi-Maxx, Fertilome F- Stop Lawn & Garden Fungicide), propiconazole (Banner MAXX, Fertilome Liquid Systemic Fungicide, Bonide Infuse Systemic Disease Control) or tebuconazole (BioAdvanced Disease Control for Roses, Flowers & Shrubs). References: 1. Powdery Mildew in the Flower Garden, University of Minnesota Extension 2. Peony Powdery Mildew, University of Illinois Extension, Home, Yard & Garden Pest Newsletter, July 16, 2010 Last Update: 1/16/2020 Brand names appearing in this publication are for product identification purposes only. No endorsement is intended, nor is criticism implied of similar products not mentioned. Kansas State University Agricultural Experiment Station and Cooperative Extension Service . -

BIOLOGY and HOST SPECIFICITY of Tectococcus
BIOLOGY AND HOST SPECIFICITY OF Tectococcus ovatus (HEMIPTERA: ERIOCOCCIDAE), A POTENTIAL BIOLOGICAL CONTROL AGENT OF THE INVASIVE STRAWBERRY GUAVA, Psidium cattleianum (MYRTACEAE), IN FLORIDA By FRANCIS JAMES WESSELS IV A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2005 Copyright 2005 by Frank J. Wessels This document is dedicated to my parents, for their support and generosity throughout my educational career. Without them, this work would not have been possible. ACKNOWLEDGMENTS I would like to thank my major professor Dr. James P. Cuda for his invaluable guidance and help throughout my degree program. I also thank my other committee members, Dr. Kenneth A. Langeland and Dr. William A. Overholt, for their comments and suggestions on my research and this manuscript. iv TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................................................................................. iv LIST OF TABLES............................................................................................................ vii LIST OF FIGURES ......................................................................................................... viii ABSTRACT....................................................................................................................... ix CHAPTER 1 INTRODUCTION ........................................................................................................1 -

Peach Volatile Emission and Attractiveness of Different Host Plant
www.nature.com/scientificreports OPEN Peach volatile emission and attractiveness of diferent host plant volatiles blends to Cydia molesta in adjacent peach and pear orchards Peng‑fei Lu1* & Hai‑li Qiao2 The oriental fruit moth (OFM), Cydia (= Grapholitha) molesta, is a highly damaging pest; peaches are its primary host, and pears serve as post‑peach secondary hosts during the late season in China. We collected volatiles from detached peach shoots and fruits, and identifed them with gas chromatography–mass spectrometry (GC–MS). Antennally active compounds were identifed by gas chromatography-electroantennogram detection (GC-EAD), and these were further tested in the laboratory and feld. We detected consistent electroantennographic activity was for ten compounds. Signifcantly more C. molesta females were caught with a mixture of female EAD-active compounds identifed from the detached matured peach fruits (nonanal, butyl acetate, 3-methylbutyl acetate, hexyl acetate, (Z)-3-hexenyl acetate, linalool and farnesene) than other mixtures mimicking the volatile profle from detached matured fruits or shoots. We identifed a new GC-EAD active mixture from intact peach shoots composed of nonanal, (Z)-3-hexenyl acetate, (E)-β-ocimene, and 6-methyl- 5-hepten-2-one. In the feld test, the background odour of orchards could afect trap catches, and two peach-derived blends together with two previously known pear-derived blends were proven to be able to monitor the seasonal OFM population dispersal in adjacent orchards. These host plant blends will be efective for further designing candidate attractants for season-long C. molesta population dynamic monitoring. Phytochemicals are important olfactory cues for moths to fnd hosts and lay eggs 1–4. -

Native Trees of Georgia.Pdf
NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION J. Frederick Allen Director Tenth Printing - 2000 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W.