Bactrocera Tryoni: Host Crops and Other Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New World Guava Fruit Fly, Anastrepha Striata, Host List the Berries, Fruit, Nuts and Vegetables of the Listed Plant Species Are Now Considered Host Articles for A
November 2018 New World Guava Fruit Fly, Anastrepha striata, Host List The berries, fruit, nuts and vegetables of the listed plant species are now considered host articles for A. striata. Unless proven otherwise, all cultivars, varieties, and hybrids of the plant species listed herein are considered suitable hosts of A. striata. Scientific Name Common Name Acca sellowiana (O. Berg) Burret Pineapple-guava, feijoa Anacardium occidentale L. Cashew, cajuil Annona cherimola Mill. Cherimoya, custard-apple Annona muricata L. Soursop, araticum-grande Averrhoa carambola L. Carambola, starfruit Bellucia dichotoma Cogn. N/A Bellucia grossularioides (L.) Triana N/A Byrsonima crassifolia (L.) Kunth Craboo, golden-spoon Calycolpus moritzianus (O. Berg) Burret N/A Campomanesia lineatifolia Ruiz & P av. Guabiroba, guayaba de leche Carica papaya L. Papaya, pawpaw 1 Citrus xsinensis (L.) Osbeck Sweet orange, blood orange Citrus xtangeloJ. W. Ingram & H. E. Moore Tangelo, uglifruit Coffea arabica L. Arabica coffee, Arabian coffee Couma utilis (Mart.) Mull. Arg. Sorva, sorva pequena Diospyros digyna Jacq. Black persimmon, black sapote Eriobotrya japonica (Thunb). Lindl. Loquat, Japanese-medlar Eugenia ligustrina (Sw.) Willd. Birchberry, privet stopper Eugenia luschnathiana (O. Berg) Klotzsch ex B. D. Jacks N/A Eugenia stipitata McVaugh Araca-boi, araza Eugenia uniflora L. Brazil-cherry, Surinam-cherry Inga edulis Mart. Ice-cream-bean, inga-cipo Inga feuilleei DC. Pacae, pacay Inga velutina Wiild. N/A Malpighia glabra L. Escobillo Mangifera indica L. Common mango, Indian mango Manilkara zapota (L.) P. Royen Sapote, naseberry, sapodilla Oenocarpus bacaba Mart. Bacaba palm Common passionfruit, purple Passiflora edulis Sims granadilla Persea americana Mill. Avocado, abacate 2 Pouteria caimito (Ruiz & Pav.) Radlk. -

9. Sterile Fly Release Densities
56 Guidance for packing, shipping, holding and release of sterile flies in area-wide fruit fly control programmes 9. Sterile fly release densities STEP V OF PROCESS IN FLOW CHART IN APPENDIX 2 9.1 FACTORS TO CONSIDER FOR ESTABLISHMENT OF STERILE FLY DENSITY (FROM HENDRICHS ET AL. 2005) 9.1.1 Pest aggregation Aside from the absolute population density, the degree of population aggregation or dispersion is important. Sterile insects are often released by aircraft, and are thus distributed fairly homogeneously over the target area, irrespective of whether the target pest is distributed evenly or clumped. Pest insects with a clumped distribution require higher release rates (Barclay 2005) as compared with a homogeneous pest distribution, to obtain the required sterile to wild male ratios (Vreysen 2005), and thus pest aggregation also affects strategy selection and its cost. Only if the released insects can find the same aggregation sites and aggregate in a similar manner as wild insects, so that adequate sterile to wild male over-flooding ratios are obtained in those sites, is there no need to increase release rates to compensate for such clumping. 9.1.2 Sterile male longevity The density of the sterile male population in the field, which fluctuates in relation to the release frequency and the sterile male mortality rate, should not decrease below that needed to maintain the critical overflooding ratio (Figure 9.1, upper graph) (Barclay 2005; Kean et al. 2005). Therefore, the frequency of release and number of sterile males released has to be carefully assessed in relation to the average longevity or survival of the sterile males, to effectively avoid periods when insufficient sterile males are present in the field (Figure 9.1, lower graph). -

The Influence of Invasive Alien Plant Control on the Foraging Habitat Quality of the Mauritian Flying Fox (Pteropus Niger)
Master’s Thesis 2017 60 ECTS Department of Ecology and Natural Resource Management The influence of invasive alien plant control on the foraging habitat quality of the Mauritian flying fox (Pteropus niger) Gabriella Krivek Tropical Ecology and Natural Resource Management ACKNOWLEDMENTS First, I would like to express my gratitude to my supervisors Torbjørn Haugaasen from INA and Vincent Florens from the University of Mauritius, who made this project possible and helped me through my whole study and during the write-up. I also want to thank to NMBU and INA for the financial support for my field work in Mauritius. I am super thankful to Maák Isti for helping with the statistical issues and beside the useful ideas and critical comments, sending me positive thoughts on hopeless days and making me believe in myself. Special thanks to Cláudia Baider from The Mauritius Herbarium, who always gave me useful advice on my drafts and also helped me in my everyday life issues. I would like to thank Prishnee for field assistance and her genuine support in my personal life as well. I am also grateful to Mr. Owen Griffiths for his permission to conduct my study on his property and to Ravi, who generously provided me transportation to the field station, whenever I needed. Super big thanks to Rikard for printing out and submitting my thesis. I am also thankful to my family for their support during my studies, fieldwork and writing, and also for supporting the beginning of a new chapter in Mauritius. Finally, a special mention goes to my love, who has been motivating me every single day not to give up on my dreams. -

Queensland Brain Institute 2017 Annual Report Director’S Message
Queensland Brain Institute 2017 Annual Report Director’s message 2017 was another year of strong achievement in our research output, as reflected in the number and quality of our peer-reviewed publications in leading scientific journals, and our grant success in both the NHMRC Project Grant and the ARC Discovery Project schemes. It was particularly pleasing that many of our younger recruits obtained grant funding. QBI’s clinical links were strengthened through a joint appointment with Mater Neuroscience; Professor Peter Nestor, a clinical neurologist from the German Center for Neurodegenerative Diseases, started in October. Refurbishment of the Ritchie building occurred during much of the year, and a number of Faculty have moved across into the new space; the breakthrough from the Clem Jones Centre for Ageing Dementia Research (CJCADR) into the Ritchie building on Level 3 is also complete, and seamlessly links the two buildings. This space forms a visible clinical interface for QBI, and will include a Memory Clinic for testing patients with neurodegenerative diseases. In August, QBI hosted the first official visit of the newly appointed International Research Review Board. Half of the Faculty were reviewed, and the research focus, current and future, of the Institute was reviewed. The remaining half are also scheduled for review in December 2018. The initial Report was very supportive, and noted that the breadth of neuroscience research was impressive, and that QBI is a world-leading Institute that enjoys high international visibility. Recommendations outlined in the Report will be examined in detail over the coming year. Professor Pankaj Sah Director, Queensland Brain Institute Queensland Brain Institute Annual Report 2017 1 Research highlights There were several major research discoveries in 2017, as well as the progression of a number of research outcomes towards commercialisation. -

Parasitoids of Queensland Fruit Fly Bactrocera Tryoni in Australia and Prospects for Improved Biological Control
Insects 2012, 3, 1056-1083; doi:10.3390/insects3041056 OPEN ACCESS insects ISSN 2075-4450 www.mdpi.com/journal/insects/ Review Parasitoids of Queensland Fruit Fly Bactrocera tryoni in Australia and Prospects for Improved Biological Control Ashley L. Zamek 1,, Jennifer E. Spinner 2 Jessica L. Micallef 1, Geoff M. Gurr 3 and Olivia L. Reynolds 4,* 1 Elizabeth Macarthur Agricultural Institute, NSW Department of Primary Industries, Woodbridge Road, Menangle, NSW 2568, Australia; E-Mails: [email protected] (A.L.Z.); [email protected] (J.L.M) 2 EH Graham Centre for Agricultural Innovation, NSW Department of Primary Industries and Charles Sturt University, Locked Bag 588, Wagga Wagga, NSW 2678, Australia; E-Mail: [email protected] 3 EH Graham Centre for Agricultural Innovation, NSW Department of Primary Industries and Charles Sturt University, Charles Sturt University, P.O. Box 883, Orange, NSW 2800, Australia; E-Mail: [email protected] 4 EH Graham Centre for Agricultural Innovation, NSW Department of Primary Industries and Charles Sturt University, Elizabeth Macarthur Agricultural Institute, Woodbridge Road, Menangle, NSW 2568, Australia Present address: Level 1, 1 Phipps Close DEAKIN ACT 2600 Australia. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +61-0-2-4640-6426; Fax: +61-0-2-4640-6300. Received: 3 September 2012; in revised form: 4 October 2012 / Accepted: 10 October 2012 / Published: 22 October 2012 Abstract: This review draws together available information on the biology, methods for study, and culturing of hymenopteran parasitoids of the Queensland fruit fly, Bactrocera tryoni, and assesses prospects for improving biological control of this serious pest. -

Plant Biometrics of Malay, Rose and Water Apple Guilherme Nacata1 & Renata Aparecida De Andrade2
ISSN 0100-2945 DOI: http://dx.doi.org /10.1590/0100-29452018131 Botanic and Physiology Plant biometrics of malay, rose and water apple Guilherme Nacata1 & Renata Aparecida de Andrade2 Abstract - Due to the lack of studies comparing Syzygium malaccensis(L) Meer & Perry, Syzygium aqueum Burm.f. and Syzygium jambos (L) Alston Syzygium in relation to the botanical genus Sygygium regarding the physical characteristics, the present research was conducted, aiming at biometrically characterizing each of the three species, as well as comparing them. The fruits were collected from the São Paulo State University (Unesp), School of Agricultural and Veterinarian Sciences, Jaboticabal. Syzygium plants were used for the evaluation of: fruit and seed masses (g); width and length of leaves (cm) and of seeds (cm); percentage of fruit pulp; length of leaf petiole (cm); and leaf area (cm2). Fifty fruits, leaves and seeds of each species were used, with 5 replicates of 10 samples each and the data were submitted to descriptive statistical analysis and organized into frequency tables. For comparative purposes among the species, a blocks randomized design was adopted, being analyzed by the Tukey Test at 5% of probability. The malay and water apple present fruits with high yield of pulp. The fruit of the malay apple are light, with narrow diameter, medium length, broad seeds, narrow and small leaves, with small leaf area. The rose apple fruit present medium mass, width and length, with broad seeds, narrow leaves, medium length and small leaf area. The water apple fruit are light, broad, long, without seeds, with broad leaves, long and having medium leaf area. -

BIOLOGY and HOST SPECIFICITY of Tectococcus
BIOLOGY AND HOST SPECIFICITY OF Tectococcus ovatus (HEMIPTERA: ERIOCOCCIDAE), A POTENTIAL BIOLOGICAL CONTROL AGENT OF THE INVASIVE STRAWBERRY GUAVA, Psidium cattleianum (MYRTACEAE), IN FLORIDA By FRANCIS JAMES WESSELS IV A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2005 Copyright 2005 by Frank J. Wessels This document is dedicated to my parents, for their support and generosity throughout my educational career. Without them, this work would not have been possible. ACKNOWLEDGMENTS I would like to thank my major professor Dr. James P. Cuda for his invaluable guidance and help throughout my degree program. I also thank my other committee members, Dr. Kenneth A. Langeland and Dr. William A. Overholt, for their comments and suggestions on my research and this manuscript. iv TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................................................................................. iv LIST OF TABLES............................................................................................................ vii LIST OF FIGURES ......................................................................................................... viii ABSTRACT....................................................................................................................... ix CHAPTER 1 INTRODUCTION ........................................................................................................1 -

White Pupae Genes in the Tephritids Ceratitis Capitata, Bactrocera Dorsalis and 2 Zeugodacus Cucurbitae: a Story of Parallel Mutations
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.08.076158; this version posted May 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 White pupae genes in the Tephritids Ceratitis capitata, Bactrocera dorsalis and 2 Zeugodacus cucurbitae: a story of parallel mutations 3 Short title: Genetic mutations causing white pupae phenotypes 4 Ward CMa,1, Aumann RAb,1, Whitehead MAc, Nikolouli Kd, Leveque G e,f, Gouvi Gd,g, Fung Eh, 5 Reiling SJe, Djambazian He, Hughes MAc, Whiteford Sc, Caceres-Barrios Cd, Nguyen TNMa,k, 6 Choo Aa, Crisp Pa,h, Sim Si, Geib Si, Marec Fj, Häcker Ib, Ragoussis Je, Darby ACc, Bourtzis 7 Kd,*, Baxter SWk,*, Schetelig MFb,* 8 9 a School of Biological Sciences, University of Adelaide, Australia, 5005 10 b Justus-Liebig-University Gießen, Institute for Insect Biotechnology, Department of Insect 11 Biotechnology in Plant Protection, Winchesterstr. 2, 35394 Gießen, Germany 12 c Centre for Genomic Research, Institute of Integrative Biology, The Biosciences Building, Crown Street, 13 Liverpool, L69 7ZB, United Kingdom 14 d Insect Pest Control Laboratory, Joint FAO/IAEA Division of Nuclear Techniques in Food and 15 Agriculture, Seibersdorf, A-1400 Vienna, Austria 16 e McGill University Genome Centre, McGill University, Montreal, Quebec, Canada 17 f Canadian Centre for Computational Genomics (C3G), McGill University, Montreal, Quebec, Canada 18 g Department of Environmental Engineering, University of Patras, 2 Seferi str., 30100 Agrinio, Greece 19 h South Australian Research and Development Institute, Waite Road, Urrbrae, South Australia 5064 20 i USDA-ARS Daniel K. -
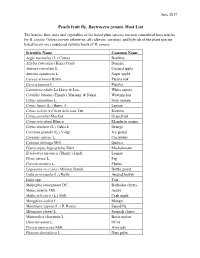
Peach Fruit Fly, Bactrocera Zonata, Host List the Berries, Fruit, Nuts and Vegetables of the Listed Plant Species Are Now Considered Host Articles for B
June 2017 Peach fruit fly, Bactrocera zonata, Host List The berries, fruit, nuts and vegetables of the listed plant species are now considered host articles for B. zonata. Unless proven otherwise, all cultivars, varieties, and hybrids of the plant species listed herein are considered suitable hosts of B. zonata. Scientific Name Common Name Aegle marmelos (L.) Correa Baeltree Afzelia xylocarpa (Kurz) Craib Doussie Annona reticulata L. Custard apple Annona squamosa L. Sugar apple Careya arborea Roxb. Patana oak Carica papaya L. Papaya Casimiroa edulis La Llave & Lex. White sapote Citrullus lanatus (Thunb.) Matsum. & Nakai Watermelon Citrus aurantium L. Sour orange Citrus limon (L.) Burm. f. Lemon Citrus nobilis x Citrus deliciosa Ten. Kinnow Citrus paradisi Macfad. Grapefruit Citrus reticulata Blanco Mandarin orange Citrus sinensis (L.) Osbeck Orange Coccinia grandis (L.) Voigt Ivy gourd Cucumis sativus L. Cucumber Cydonia oblonga Mill. Quince Elaeocarpus hygrophilus Kurz Ma-kok-nam Eriobotrya japonica (Thunb.) Lindl. Loquat Ficus carica L. Fig Grewia asiatica L. Phalsa Lagenaria siceraria (Molina) Standl. Bottle gourd Luffa acutangula (L.) Roxb. Angled loofah Luffa spp. Tori Malpighia emarginata DC. Barbados cherry Malus pumila Mill. Apple Malus sylvestris (L.) Mill. Crab apple Mangifera indica L. Mango Manilkara zapota (L.) P. Royen Sapodilla Mimusops elengi L. Spanish cherry Momordica charantia L. Bitter melon Olea europaea L. Olive Persea americana Mill. Avocado Phoenix dactylifera L. Date palm Prunus armeniaca L. Apricot Prunus avium (L.) L. Sweet cherry Prunus domestica L. Plum Prunus persica (L.) Batsch Peach Prunus sp. N/A Psidium cattleyanum Sabine Strawberry guava Psidium guajava L. Guava Punica granatum L. Pomegranate Putranjiva roxburghii Wall. -

(Bactrocera) Tryoni (Queensland Fruit Fly) Tania Yonow Harvestchoice, Instepp, University of Minnesota, St
SEPTEMBER 2014 Bactrocera (Bactrocera) tryoni (Queensland Fruit Fly) Tania Yonow HarvestChoice, InSTePP, University of Minnesota, St. Paul, MN, USA CSIRO, Biosecurity and Agriculture Flagships, Canberra, Australia Information Taken From Introduction Yonow, T. and Sutherst, R.W. (1998). The geographical Bactrocera tryoni is widely recognised as one of distribution of the Queensland fruit fly, Bactrocera Australia’s worst economic pests of fruit (e.g. Clarke et al. (Dacus) tryoni, in relation to climate. Australian Journal 2011). Apart from lowering production and making fruit of Agricultural Research 49: 935–953. inedible, it has severe effects on trade to sensitive local and international markets. A number of management Background Information zones have been established to protect horticultural production areas from this species. Common Names: Queensland Fruit Fly; QFF, QFly Known Distribution Scientific Name: Bactrocera tryoni occurs in eastern parts of Australia Bactrocera tryoni (Froggatt) (http://www.ala.org.au). It also occurs in French Polynesia, New Caledonia, Pacific Islands, and Vanuatu Synonyms: (http://www.spc.int/Pacifly). See also Clarke et al. Bactrocera (Bactrocera) tryoni (Froggatt); Chae- (2011) and Dominiak and Daniels (2012) for a review of todacus sarcocephali Tryon; Chaetodacus tryoni the distribution of B. tryoni. (Froggatt); Dacus ferrugineus tryoni (Froggatt); Dacus tryoni (Froggatt); Strumeta melas Perkins & May; Description and Biology Strumeta tryoni (Froggatt); Tephritis tryoni Froggatt Adult B. tryoni are about 7 mm long and brownish in Taxonomy: colour, with distinctive yellow markings (Figure 1). Kingdom: Animalia; Phylum: Arthropoda; Females lay their eggs into soft and ripening host fruit. Class: Insecta; Order: Diptera; Family: Tephritidae Larvae (maggots - up to 10 mm long) emerge from the eggs and cause damage by living and feeding within the Crop Hosts: fruit, which may appear intact from the outside. -

CARIBBEAN REGION - NWPL 2014 FINAL RATINGS User Notes: 1) Plant Species Not Listed Are Considered UPL for Wetland Delineation Purposes
CARIBBEAN REGION - NWPL 2014 FINAL RATINGS User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps region. -

Fruits of the Fall Season Presentation
Fruits of the Fall Season *APPLE Malus pumila Fruiting Spurs -Apple *APPLE Malus pumila Woolly Apple Aphid • The infests woody parts of apple roots and limbs, often near pruning wounds, and can cause overall tree decline if roots are infested for several years. Powdery Mildew Pear Pear Fruiting Spurs - Pear EUROPEAN PEAR Pyrus communis ASIAN PEAR Pyrus pyrifolia HYBRID PEAR /SOUTHERN CROSS PEAR Pyrus X lecontei Sawflies Pear Sawfly (Pear Slug) QUINCE Cydonia oblonga QUINCE Cydonia oblonga Codling Moth • The larvae of the codling moth (Cydia pomonella) is the common apple worm. • It is native to Europe and was introduced to North America, where it has become one of the regular pests of apple orchards. It is found almost worldwide. • It also attacks pears, quince, walnuts and other tree fruits. • Codling moth adults are about 1/2 to 3/4 inch long with mottled gray wings that are held tentlike over their bodies. Their appearance blends well with most tree bark, making them difficult to detect. • If you are trapping the adults, codling moths can be distinguished from other moths by the dark, coppery brown band at the tip of their wings. Codling Moth • Codling moths overwinter as full-grown larvae within thick, silken cocoons under loose scales of bark and in soil or debris around the base of the tree. • The larvae pupate inside their cocoons in early spring and emerge as adult moths mid-March to early April. • After mating each female deposits 30 to 70 tiny, disc-shaped eggs singly on fruit, nuts, leaves, or spurs. After the eggs hatch, young larvae seek out and bore into fruit or developing nuts.