Tagging Narwhal in Greenland. Photo: Carsten Egevang/ARC-PIC.Com 175 4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Complete Mitochondrial Genome Sequences of the Arctic Ocean Codwshes Arctogadus Glacialis and Boreogadus Saida Reveal Oril and Trna Gene Duplications
Polar Biol (2008) 31:1245–1252 DOI 10.1007/s00300-008-0463-7 ORIGINAL PAPER Complete mitochondrial genome sequences of the Arctic Ocean codWshes Arctogadus glacialis and Boreogadus saida reveal oriL and tRNA gene duplications Ragna Breines · Anita Ursvik · Marianne Nymark · Steinar D. Johansen · Dag H. Coucheron Received: 4 December 2007 / Revised: 16 April 2008 / Accepted: 5 May 2008 / Published online: 27 May 2008 © The Author(s) 2008 Abstract We have determined the complete mitochon- Introduction drial genome sequences of the codWshes Arctogadus gla- cialis and Boreogadus saida (Order Gadiformes, Family More than 375 complete sequenced mitochondrial genomes Gadidae). The 16,644 bp and 16,745 bp mtDNAs, respec- from ray-Wnned Wshes have so far (December 2007) been tively, contain the same set of 37 structural genes found in submitted to the database (http://www.ncbi.nlm.nih.gov), all vertebrates analyzed so far. The gene organization is and many of these sequences have contributed considerably conserved compared to other Gadidae species, but with one to resolving phylogenetic relationships among Wshes. Evo- notable exception. B. saida contains heteroplasmic rear- lutionary relationships at diVerent taxonomic levels have rangement-mediated duplications that include the origin of been addressed, including Division (Inoue et al. 2003; Miya light-strand replication and nearby tRNA genes. Examina- et al. 2003), Subdivision (Ishiguro et al. 2003), Genus tion of the mitochondrial control region of A. glacialis, (Doiron et al. 2002; Minegishi et al. 2005), and Species B. saida, and four additional representative Gadidae genera (Yanagimoto et al. 2004; Ursvik et al. 2007). identiWed a highly variable domain containing tandem The circular mitochondrial genomes from ray-Wnned repeat motifs in A. -

Appendix 13.2 Marine Ecology and Biodiversity Baseline Conditions
THE LONDON RESORT PRELIMINARY ENVIRONMENTAL INFORMATION REPORT Appendix 13.2 Marine Ecology and Biodiversity Baseline Conditions WATER QUALITY 13.2.1. The principal water quality data sources that have been used to inform this study are: • Environment Agency (EA) WFD classification status and reporting (e.g. EA 2015); and • EA long-term water quality monitoring data for the tidal Thames. Environment Agency WFD Classification Status 13.2.2. The tidal River Thames is divided into three transitional water bodies as part of the Thames River Basin Management Plan (EA 2015) (Thames Upper [ID GB530603911403], Thames Middle [ID GB53060391140] and Thames Lower [ID GB530603911401]. Each of these waterbodies are classified as heavily modified waterbodies (HMWBs). The most recent EA assessment carried out in 2016, confirms that all three of these water bodies are classified as being at Moderate ecological potential (EA 2018). 13.2.3. The Thames Estuary at the London Resort Project Site is located within the Thames Middle Transitional water body, which is a heavily modified water body on account of the following designated uses (Cycle 2 2015-2021): • Coastal protection; • Flood protection; and • Navigation. 13.2.4. The downstream extent of the Thames Middle transitional water body is located approximately 12 km downstream of the Kent Project Site and 8 km downstream of the Essex Project Site near Lower Hope Point. Downstream of this location is the Thames Lower water body which extends to the outer Thames Estuary. 13.2.5. A summary of the current Thames Middle water body WFD status is presented in Table A13.2.1, together with those supporting elements that do not currently meet at least Good status and their associated objectives. -

Trophic Niche Partitioning of Littoral Fish Species from the Rocky Intertidal Of
Helgol Mar Res (2015) 69:385–399 DOI 10.1007/s10152-015-0444-5 ORIGINAL ARTICLE Trophic niche partitioning of littoral fish species from the rocky intertidal of Helgoland, Germany 1,2 3 4 1 N. N. Hielscher • A. M. Malzahn • R. Diekmann • N. Aberle Received: 30 January 2015 / Revised: 1 October 2015 / Accepted: 15 October 2015 / Published online: 27 October 2015 Ó Springer-Verlag Berlin Heidelberg and AWI 2015 Abstract During a 3-year field study, interspecific and the littoral zones of Helgoland and on complex benthic interannual differences in the trophic ecology of littoral fish food webs in general. species were investigated in the rocky intertidal of Hel- goland island (North Sea). We investigated trophic niche Keywords Fatty acids Á Stable isotopes Á Ctenolabrus partitioning of common coexisting littoral fish species rupestris Á Pomatoschistus minutus Á Pomatoschistus based on a multi-tracer approach using stable isotope and pictus Á Myoxocephalus scorpius Á Taurulus bubalis Á fatty acids in order to show differences and similarities in Trophic ecology Á Feeding ecology resource use and feeding modes. The results of the dual- tracer approach showed clear trophic niche partitioning of the five target fish species, the goldsinny wrasse Cteno- Introduction labrus rupestris, the sand goby Pomatoschistus minutus, the painted goby Pomatoschistus pictus, the short-spined Understanding the trophic interactions in complex and sea scorpion Myoxocephalus scorpius and the long-spined diverse marine ecosystems is a significant challenge. In sea scorpion Taurulus bubalis. Both stable isotopes and order to obtain a profound knowledge on complex fatty acids showed distinct differences in the trophic ecosystems, it is important to address trophodynamic ecology of the studied fish species. -

Biodiversity of Arctic Marine Fishes: Taxonomy and Zoogeography
Mar Biodiv DOI 10.1007/s12526-010-0070-z ARCTIC OCEAN DIVERSITY SYNTHESIS Biodiversity of arctic marine fishes: taxonomy and zoogeography Catherine W. Mecklenburg & Peter Rask Møller & Dirk Steinke Received: 3 June 2010 /Revised: 23 September 2010 /Accepted: 1 November 2010 # Senckenberg, Gesellschaft für Naturforschung and Springer 2010 Abstract Taxonomic and distributional information on each Six families in Cottoidei with 72 species and five in fish species found in arctic marine waters is reviewed, and a Zoarcoidei with 55 species account for more than half list of families and species with commentary on distributional (52.5%) the species. This study produced CO1 sequences for records is presented. The list incorporates results from 106 of the 242 species. Sequence variability in the barcode examination of museum collections of arctic marine fishes region permits discrimination of all species. The average dating back to the 1830s. It also incorporates results from sequence variation within species was 0.3% (range 0–3.5%), DNA barcoding, used to complement morphological charac- while the average genetic distance between congeners was ters in evaluating problematic taxa and to assist in identifica- 4.7% (range 3.7–13.3%). The CO1 sequences support tion of specimens collected in recent expeditions. Barcoding taxonomic separation of some species, such as Osmerus results are depicted in a neighbor-joining tree of 880 CO1 dentex and O. mordax and Liparis bathyarcticus and L. (cytochrome c oxidase 1 gene) sequences distributed among gibbus; and synonymy of others, like Myoxocephalus 165 species from the arctic region and adjacent waters, and verrucosus in M. scorpius and Gymnelus knipowitschi in discussed in the family reviews. -

APPENDIX 1 Classified List of Fishes Mentioned in the Text, with Scientific and Common Names
APPENDIX 1 Classified list of fishes mentioned in the text, with scientific and common names. ___________________________________________________________ Scientific names and classification are from Nelson (1994). Families are listed in the same order as in Nelson (1994), with species names following in alphabetical order. The common names of British fishes mostly follow Wheeler (1978). Common names of foreign fishes are taken from Froese & Pauly (2002). Species in square brackets are referred to in the text but are not found in British waters. Fishes restricted to fresh water are shown in bold type. Fishes ranging from fresh water through brackish water to the sea are underlined; this category includes diadromous fishes that regularly migrate between marine and freshwater environments, spawning either in the sea (catadromous fishes) or in fresh water (anadromous fishes). Not indicated are marine or freshwater fishes that occasionally venture into brackish water. Superclass Agnatha (jawless fishes) Class Myxini (hagfishes)1 Order Myxiniformes Family Myxinidae Myxine glutinosa, hagfish Class Cephalaspidomorphi (lampreys)1 Order Petromyzontiformes Family Petromyzontidae [Ichthyomyzon bdellium, Ohio lamprey] Lampetra fluviatilis, lampern, river lamprey Lampetra planeri, brook lamprey [Lampetra tridentata, Pacific lamprey] Lethenteron camtschaticum, Arctic lamprey] [Lethenteron zanandreai, Po brook lamprey] Petromyzon marinus, lamprey Superclass Gnathostomata (fishes with jaws) Grade Chondrichthiomorphi Class Chondrichthyes (cartilaginous -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -
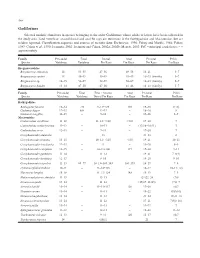
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Kane-Higham-2012.Pdf
G Model ZOOL-25301; No. of Pages 10 ARTICLE IN PRESS Zoology xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Zoology journa l homepage: www.elsevier.com/locate/zool Life in the flow lane: differences in pectoral fin morphology suggest transitions in station-holding demand across species of marine sculpin ∗ Emily A. Kane , Timothy E. Higham University of California, Riverside, Department of Biology, 900 University Ave., Riverside, CA 92521, USA a r t i c l e i n f o a b s t r a c t Article history: Aquatic organisms exposed to high flow regimes typically exhibit adaptations to decrease overall drag Received 28 September 2011 and increase friction with the substrate. However, these adaptations have not yet been examined on a Received in revised form 27 February 2012 structural level. Sculpins (Scorpaeniformes: Cottoidea) have regionalized pectoral fins that are modified Accepted 7 March 2012 for increasing friction with the substrate, and morphological specialization varies across species. We examined body and pectoral fin morphology of 9 species to determine patterns of body and pectoral Keywords: fin specialization. Intact specimens and pectoral fins were measured, and multivariate techniques deter- Benthic fishes mined the differences among species. Cluster analysis identified 4 groups that likely represent differences Scorpaeniformes in station-holding demand, and this was supported by a discriminant function analysis. Primarily, the Flow regime high-demand group had increased peduncle depth (specialization for acceleration) and larger pectoral Functional regionalization Station-holding fins with less webbed ventral rays (specialization for mechanical gripping) compared to other groups; secondarily, the high-demand group had a greater aspect ratio and a reduced number of pectoral fin rays (specialization for lift generation) than other groups. -

Descriptive Key to the Otoliths of Gadid Fishes of the Bering, Chukchi, and Beaufort Seas KATHRYN J
ARCTIC VOL. 34, NO. 1 (MARCH 1981), P. 55-59 Descriptive Key to the Otoliths of Gadid Fishes of the Bering, Chukchi, and Beaufort Seas KATHRYN J. FROST’ ABSTRACT. An illustrated key with supplementary descriptive material is presented for six species or species groups of gadid fishes which are of trophic importance in the Bering, Chukchi, and Beaufort seas. These species include: Arcrogadus spp. Djagin, Boreogadus saida (Lepechin), Eleginus gracilis (Tilesius), Gadus macrocephalus Tilesius, Microgadus proximus (Girard), and Theragra chalcogramma (Pallas). RESUME. Une clC d’identificationillustree par des figures avec un complement descriptif est ici presentee pour six espbces ou groupes d’espbces de poissons de la famiile des gadidCs, lesquels ont une importance au point de vue trophique dans les mers de BCring, des Tchouktches et deBeaufort. Ces espbcescomprennent: Arctogadus spp. Djagin, Boreogadus saida (Lepechin), Eleginus gracilis (Tilesius), Gadus macrocephalus Tilesius, Microgadus proximus (Girard), et Theragra chalcogramma (Pallas). Traduit par Jean-Guy Brossard, Laboratoire d’Archeologie de I’Universit6 due Quebec A Montreal. Key words: otolith, gadid fishes, Arctogadus, Boreogadus, Eleginus, Gadus, Microgadus, Theragra INTRODUCTION size or when certain features vary such that an otolith of Investigations of food habits of marine animals almost one species closelyresembles that of other species. Furth- invariably involve analysisof stomach contents. Success- er, keys,are often usedby readers who have little familiar- ful stomach contents analysis usually requires that prey ity with otoliths and limited access to comparative mate- items be recognized by characteristic fragments. In this rial, and who therefore require more detailed descriptive respect the sagittal otoliths of bony fishes are very useful material. -

The Ringed Seal, Phoca Hispida, of the Canadian Western Arctic Jimmy Memorana of Holman, Northwest Territories, with a Day's Catch of Ringed Seals
Canadian Bulletin of Fisheries_and Aquatic-Sciences 216 DFO - Lib ary / MPO - B b otheque 11111 I 1111 11111 11 11 12039511 The Ringed Seal, Phoca hispida. of the Canadian Western Arctic Thomas G. Smith -SW D23 92/3 2 C. / Fisheries Pêches 1+ and Oceans et Océans The Ringed Seal, Phoca hispida, of the Canadian Western Arctic Jimmy Memorana of Holman, Northwest Territories, with a day's catch of ringed seals. Il Canadian Bulletin of Fisheries and Aquatic Sciences 216 The Ringed Seal, Phoca hispida, of the Canadian Western Arctic Thomas G. Smith Department of Fisheries and Oceans Arctic Biological Station 555 St-Pierre Blvd. Sainte-Anne-de-Bellevue, Quebec H9X 3R4 DEPARTMENT OF FISHERIES AND OCEANS Ottawa 1987 III The Canadian Bulletins of Fisheries and Aquatic. Sciences are designed to interpret current knowledge in scientific fields pertinent to Canadian fisheries and aquatic environments. The Canadian Journal of Fisheries and Aquatic Sciences is published in annual volumes of monthly issues. Canadian Special Publications of Fisheries and Aquatic Sciences arc issued periodically. These series are available from authorized bookstore agents and other bookstores, or you may send your prepaid order to the Canadian Government Publishing Centre, Supply and Services Canada, Ottawa, Ont. KIA 0S9. Make cheque or money order payable in Canadian funds to the Receiver General for Canada. Information and Publications Branch Dixi Lambert Director General Johanna M. Reinhart, M.Sc. Director and Editor Gerald J. Neville Editorial and Publishing Services Editorial Office: Department of Fisheries and Oceans Communications Directorate Information and Publications Branch 200 Kent Street Ottawa, Ontario, Canada K IA 0E6 Typesetter: K.G. -

Baseline Respiration and Spontaneous Activity of Sluggish Marine Tropical Fish of the Family Scorpaenidae
MARINE ECOLOGY PROGRESS SERIES Vol. 219: 229–239, 2001 Published September 10 Mar Ecol Prog Ser Baseline respiration and spontaneous activity of sluggish marine tropical fish of the family Scorpaenidae Christopher Zimmermann1, 2,*, Andreas Kunzmann1, 3 1Institut für Polarökologie der Universität Kiel, Wischhofstr. 1–3, Geb. 12, 24148 Kiel, Germany 2Institut für Seefischerei, Bundesforschungsanstalt für Fischerei, Palmaille 9, 22767 Hamburg, Germany 3Zentrum für Marine Tropenökologie, Universität Bremen, Fahrenheitstr. 6, 28359 Bremen, Germany ABSTRACT: Baseline respiration and spontaneous activity were determined simultaneously for 6 specimens of tropical scorpionfishes, belonging to 2 different genera: Scorpaenopsis oxycephalus and S. diabolus (false stonefish) and Parascorpaena sp. and P. aurita. The experiments were conducted in an intermittent-flow respirometer at 24°C, at ambient light regime (12 h dark: 12 h light) and salinity (S = 32). Permanent measurements of fish activity by an infrared video system (number of move- ments) and oxygen consumption were coupled allowing the calculation of a standard oxygen con- sumption (SOC) rate. The experimental set-up allowed quantification of spontaneous activity. Rela- –1 –1 tive SOC values varied between 32.3 mg O2 h kg wet mass (wm) for a large S. oxycephalus (82 g –1 –1 WM) and 68.9 mg O2 h kg WM for the smallest investigated specimen, a P. aurita (16 g WM). The latter also showed the highest spontaneous activity rate (mean 98 movements h–1; max.: 870 h–1), while the values for the most sluggish individual did not exceed 24.6 h–1 (mean) and 480 h–1 (max.), –1 respectively. Absolute SOC for a standard mass of 50 g was calculated to be 2.0 mg O2 h , using a –1 0.527 wet mass versus oxygen consumption relationship of SOC (mg O2 h ) = 0.26 × WM(g) . -

Resource Partitioning Among Myoxocephalus Sculpins, and Their Predator–Prey Relationships with Chionoecetes Crabs in the Eastern Bering Sea
Vol. 464: 221–235, 2012 MARINE ECOLOGY PROGRESS SERIES Published September 19 doi: 10.3354/meps09878 Mar Ecol Prog Ser Resource partitioning among Myoxocephalus sculpins, and their predator–prey relationships with Chionoecetes crabs in the eastern Bering Sea Todd T. TenBrink*, Troy W. Buckley Resource Ecology and Fisheries Management Division, Alaska Fisheries Science Center, National Marine Fisheries Service, NOAA, 7600 Sand Point Way NE, Seattle, Washington 98115, USA ABSTRACT: Interspecific and intraspecific variation in the distributions and diets of congeneric sculpins, Myoxocephalus jaok, M. polyacanthocephalus, and M. scorpius, were described from data collected during summer trawl surveys of the eastern Bering Sea (EBS) continental shelf between 2000 and 2010 by the Alaska Fisheries Science Center (AFSC). Generalized additive models (GAMs) of abundance (count) and presence-absence data were applied separately for each species to examine the influence of selected factors (depth, bottom temperature, substrate type and location) on distribution and abundance. The final models indicated strong predictive relationships, suggesting habitat partitioning between species. Ontogenetic distributions were observed as larger individuals of each species tended to be associated with deeper depths and lower latitudes. Analysis of stomach contents indicated that these species consume a wide variety of prey and that the diet composition also shifted ontogenetically. Multivariate analyses of the mean percent weight (%W) of 23 diet categories indicated that the diets differed among and within species. The diet of M. jaok consisted primarily of mysids, shrimps, and flatfish, with larger sculpins feeding on a greater amount of flatfish prey and less benthic infauna. Both M. polyacan- thocephalus and M. scorpius fed considerably on commercially important Chionoecetes crabs, particularly C.