Savene, INN-Dexrazoxane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
AMIFOSTINE for INJECTION Incidence of Grade 2 Or Higher Xerostomia (RTOG Criteria)
TABLE 4 AMIFOSTINE FOR INJECTION Incidence of Grade 2 or Higher Xerostomia (RTOG criteria) Amifostine for RT p-value only Injection +RT LBL-7062PD Acute DESCRIPTION 51% (75/148) 78% (120/153) p<0.0001 ( 90 days from Amifostine for Injection is an organic thiophosphate cytoprotective agent known chemically ɖ start of radiation) as 2-[(3-aminopropyl)amino]ethanethiol dihydrogen phosphate (ester) and has the following structural formula: Latea 35% (36/103) 57% (63/111) p=0.0016 (9-12 months H2N(CH2)3NH(CH2)2S-PO3H2 post radiation) Amifostine is a white crystalline powder which is freely soluble in water. Its empirical aBased on the number of patients for whom actual data were available. formula is C5H15N2O3PS and it has a molecular weight of 214.22. Amifostine for Injection is the trihydrate form of amifostine and is supplied as a sterile At one year following radiation, whole saliva collection following radiation showed that lyophilized powder requiring reconstitution for intravenous infusion. Each single-use 10 mL more patients given Amifostine for Injection produced >0.1 gm of saliva (72% vs. 49%). vial contains 500 mg of amifostine on the anhydrous basis. In addition, the median saliva production at one year was higher in those patients who CLINICAL PHARMACOLOGY received amifostine (0.26 gm vs. 0.1 gm). Stimulated saliva collections did not show Amifostine is a prodrug that is dephosphorylated by alkaline phosphatase in tissues to a a difference between treatment arms. These improvements in saliva production were pharmacologically active free thiol metabolite. This metabolite is believed to be responsible supported by the patients’ subjective responses to a questionnaire regarding oral dryness. -

Amifostine Is a Nephro-Protectant in Patients Receiving Treatment with Cisplatin- Myth, Mystery Or Matter-Of-Fact?
Ha SS, Rubaina K, Lee CS, John V, Seetharamu N. Amifostine is a Nephro-Protectant in Patients Receiving Treatment with Cisplatin- Myth, Mystery or Matter-of-Fact?. J Nephrol Sci.2021;3(1):4-8 Review Article Open Access Amifostine is a Nephro-Protectant in Patients Receiving Treatment with Cisplatin- Myth, Mystery or Matter-of-Fact? Sin Sil Ha1, Kazi Rubaina1, Chung-Shien Lee1,2, Veena John2, Nagashree Seetharamu2* 1St. John’s University, College of Pharmacy and Health Sciences, Department of Clinical Health Professions, 8000 Utopia Parkway, Queens, NY 11439, USA. 2Division of Medical Oncology and Hematology, Northwell Health Cancer Institute, Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, 450 Lakeville Road, Lake Success, NY 11042, USA. Article Info Abstract Article Notes Despite reports of amifostine possibly protecting nephrotoxicity from Received: December 22, 2020 cisplatin, it has not been recommended by any guidelines committees or Accepted: February 01, 2021 routinely prescribed in clinical practice over the past decade. In this article, *Correspondence: we review literature and guidelines regarding use of amifostine in oncology *Dr. Nagashree Seetharamu, Division of Medical Oncology practice for protection against adverse effects from certain chemotherapeutic and Hematology, Northwell Health Cancer Institute, Donald agents, in particular as a nephro-protectant in patients receiving cisplatin. & Barbara Zucker School of Medicine at Hofstra/Northwell, 450 Lakeville Road, Lake Success, NY 11042, USA; Email: [email protected]. Background Information ©2021 Seetharamu N. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License. Amifostine (Ethyol) is a prodrug that is dephosphorylated by alkaline phosphatase in tissues to a pharmacologically active free Keywords thiol metabolite. -

Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Acla
[CANCER RESEARCH 49. 4357-4362, August 1. 1989] Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Aclarubicin Hydrochloride 1omonimi Ichihara,1 Kiyoshi Sakamoto, Katsutaka Mori, and Masanobu Akagi Department of Surgery II, Kumamoto University Medical School, Kumamoto 860, Japan 4BSTRACT MATERIALS AND METHODS Transcatheter arterial Chemoembolization therapy using polylactic Preparation of PLA-ACRms acid microspheres containing aclarubicin hydrochloride (ACR) was per PLA-ACRms were prepared in the pharmacy of the Kumamoto formed in 62 patients with primary hepatocellular carcinoma. These microspheres were about 200 ¡anin diameter and contained 10% (w/w) University Hospital. Briefly, aclarubicin hydrochloride and isopropyl aclarubicin. A single dose of polylactic acid microspheres containing ACR myristate, a medium-chain fatty acid ester, were dissolved in 7.5% (50-100 mg of ACR) was administered 1 to 8 times with a mean of 2.2 polylactic acid-methylene chloride. The resultant solution was dispersed in 1% gelatin solution and sterilized in an autoclave for 20 min at doses (a total of 160 treatments) in 62 patients. Antitumor effects were 120°C;thismixture was then stirred with a magnetic stirrer at 500 rpm observed from the decrease in serum a-fetoprotein levels (82.1% of the patients) and in two dimensional size of tumor on computed tomography for 1 h. The resultant microspheres were collected by filtration through (93.6%). The cumulative survival rate was 54.3% at 1 year, 24.6% at 2 a membrane filter (3 //m pore diameter), rinsed 2 to 3 times (in 1 liter years, and 19.2% at 3 years, respectively, among 59 patients with of distilled water), and dried under reduced pressure for 5 days. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

Thames Valley Chemotherapy Regimens Sarcoma
Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Chemotherapy Regimens– Sarcoma 1 of 98 Thames Valley Notes from the editor All chemotherapy regimens, and associated guidelines eg antiemetics and dose bands are available on the Network website www.tvscn.nhs.uk/networks/cancer-topics/chemotherapy/ Any correspondence about the regimens should be addressed to: Sally Coutts, Cancer Pharmacist, Thames Valley email: [email protected] Acknowledgements These regimens have been compiled by the Network Pharmacy Group in collaboration with key contribution from Prof Bass Hassan, Medical Oncologist, OUH Dr Sally Trent, Clinical Oncologist, OUH Dr James Gildersleve, Clinical Oncologist, RBFT Dr Sarah Pratap, Medical Oncologist, OUH Dr Shaun Wilson, TYA - Paediatric Oncologist, OUH Catherine Chaytor, Cancer Pharmacist, OUH Varsha Ormerod, Cancer Pharmacist, OUH Kristen Moorhouse, Cancer Pharmacist, OUH © Thames Valley Cancer Network. All rights reserved. Not to be reproduced in whole or in part without the permission of the copyright owner. Chemotherapy Regimens– Sarcoma 2 of 98 Thames Valley Thames Valley Chemotherapy Regimens Sarcoma Network Chemotherapy Regimens used in the management of Sarcoma Date published: January 2019 Date of review: June 2022 Chemotherapy Regimens Name of regimen Indication Page List of amendments to this version 5 Imatinib GIST 6 Sunitinib GIST 9 Regorafenib GIST 11 Paclitaxel weekly (Taxol) Angiosarcoma 13 AC Osteosarcoma 15 Cisplatin Imatinib – if local Trust funding agreed Chordoma 18 Doxorubicin Sarcoma 21 -

Towards the Reduction of Occupational Exposure To
TOWARDS THE REDUCTION OF OCCUPATIONAL EXPOSURE TO CYTOTOXIC DRUGS by Cristian Vasile Barzan B.Sc. Simon Fraser University, 2007 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE in The Faculty of Graduate Studies (Occupational and Environmental Hygiene) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2010 © Cristian Vasile Barzan, 2010 Abstract Background : One of the most powerful and widely used techniques in cancer treatment is the use of cytotoxic drugs in chemotherapy. These drugs are inherently hazardous with many of them causing carcinogenic, mutagenic or teratogenic health outcomes. Occupational exposure to cytotoxic drugs is of great concern due to their lack of selectivity between healthy and unhealthy cells. Widespread cytotoxic drug contamination has been reported in North America, Europe and Australia. Current cleaning protocols for hazardous antineoplastic drugs include the use of disinfectants and oxidizing agents, such as household bleach. Aim : The thesis project focused on two objectives: 1) hypothesize and confirm potential hazardous by- products arising from cleaning cyclophosphamide, a widely used cytotoxic drugs, with household bleach, a commonly used cleaning agent; 2) develop an effective and safe cleaning agent for cytotoxic drugs in order to prevent and eliminate exposure to these drugs. Methods : The gas chromatograph mass spectrum (GC/MS) was used to analyze the decomposition of cyclophosphamide by household bleach (5.25% hypochlorite). The reaction was conducted in a test-tube and the by-products extracted and derivatized prior to analysis. Multiple cleaning agent compositions were tested on 10x10cm stainless steel plates spiked with the two model cytotoxic drugs, cyclophosphamide and methotrexate. -

Cardiotoxicity of Doxorubicin Is Mediated Through Mitochondrial Iron Accumulation
Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa, … , Tejaswitha Jairaj Naik, Hossein Ardehali J Clin Invest. 2014;124(2):617-630. https://doi.org/10.1172/JCI72931. Research Article Cardiology Doxorubicin is an effective anticancer drug with known cardiotoxic side effects. It has been hypothesized that doxorubicin- dependent cardiotoxicity occurs through ROS production and possibly cellular iron accumulation. Here, we found that cardiotoxicity develops through the preferential accumulation of iron inside the mitochondria following doxorubicin treatment. In isolated cardiomyocytes, doxorubicin became concentrated in the mitochondria and increased both mitochondrial iron and cellular ROS levels. Overexpression of ABCB8, a mitochondrial protein that facilitates iron export, in vitro and in the hearts of transgenic mice decreased mitochondrial iron and cellular ROS and protected against doxorubicin-induced cardiomyopathy. Dexrazoxane, a drug that attenuates doxorubicin-induced cardiotoxicity, decreased mitochondrial iron levels and reversed doxorubicin-induced cardiac damage. Finally, hearts from patients with doxorubicin-induced cardiomyopathy had markedly higher mitochondrial iron levels than hearts from patients with other types of cardiomyopathies or normal cardiac function. These results suggest that the cardiotoxic effects of doxorubicin develop from mitochondrial iron accumulation and that reducing mitochondrial iron levels protects against doxorubicin- induced cardiomyopathy. Find the latest version: https://jci.me/72931/pdf Research article Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation Yoshihiko Ichikawa,1 Mohsen Ghanefar,1 Marina Bayeva,1 Rongxue Wu,1 Arineh Khechaduri,1 Sathyamangla V. Naga Prasad,2 R. Kannan Mutharasan,1 Tejaswitha Jairaj Naik,1 and Hossein Ardehali1 1Feinberg Cardiovascular Institute, Northwestern University School of Medicine, Chicago, Illinois, USA. -

Functional Similarity of Anticancer Drugs by MTT Bioassay
cer Scien an ce C & f o T l h a e Hiwasa et al., J Cancer Sci Ther 2011, 3.10 n r a Journal of r p u 1000099 y o J DOI: 10.4172/1948-5956. ISSN: 1948-5956 Cancer Science & Therapy Rapid Communication Open Access Functional Similarity of Anticancer Drugs by MTT Bioassay Takaki Hiwasa1*,Takanobu Utsumi1, Mari Yasuraoka1, Nana Hanamura1, Hideaki Shimada2, Hiroshi Nakajima3, Motoo Kitagawa4, Yasuo Iwadate4,5, Ken-ichiro Goto6, Atsushi Takeda7, Kenzo Ohtsuka8, Hiroyoshi Ariga9 and Masaki Takiguchi1 1Department of Biochemistry, and Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 2Department of Surgery, School of Medicine, Toho University, Ota-ku, Tokyo 143-8541, Japan 3Department of Molecular Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 4Department of Molecular and Tumor Pathology, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 5Department of Neurological Surgery, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 6Department of Orthopaedic Surgery, National Hospital Organization, Shimoshizu Hospital, Yotsukaido, Chiba 284-0003, Japan 7Laboratory of Biochemistry, Graduate School of Nutritional Sciences, Sagami Women’s University, Sagamihara, Kanagawa 252-0383, Japan 8Laboratory of Cell & Stress Biology, Department of Environmental Biology, Chubu University, Matsumoto-cho, Kasugai, Aichi 487-8501, Japan 9Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo 060-0812, Japan Abstract We prepared normal or Ha-ras-transformed NIH3T3 cells transfected stably or transiently with various tumor- related genes. The chemosensitivity of the transfected clones to 16 anticancer drugs was compared to the parental control cells using the MTT assay. -

ZINECARD® (Dexrazoxane) for Injection Regimens
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------DOSAGE FORMS AND STRENGTHS------------- These highlights do not include all the information needed to use 250 mg or 500 mg single dose vials as sterile, pyrogen-free lyophilizates. (3) ZINECARD safely and effectively. See full prescribing information for ZINECARD. -------------------------------CONTRAINDICATIONS------------------------------ ZINECARD should not be used with non-anthracycline chemotherapy ZINECARD® (dexrazoxane) for injection regimens. (4) Initial U.S. Approval: 1995 -----------------------WARNINGS AND PRECAUTIONS------------------------ ---------------------------INDICATIONS AND USAGE----------------------- Myelosuppression: ZINECARD may increase the myelosuppresive ZINECARD is a cytoprotective agent indicated for reducing the incidence and effects of chemotherapeutic agents. Perform hematological monitoring. severity of cardiomyopathy associated with doxorubicin administration in (5.1) women with metastatic breast cancer who have received a cumulative Embryo-Fetal Toxicity: Can cause fetal harm. Advise female patients of doxorubicin dose of 300 mg/m2 and who will continue to receive doxorubicin reproductive potential of the potential hazard to the fetus. (5.5, 8.1) therapy to maintain tumor control. Do not use ZINECARD with doxorubicin initiation. (1) ------------------------------ADVERSE REACTIONS------------------------------- In clinical studies, ZINECARD was administered to patients also receiving -----------------------DOSAGE AND ADMINISTRATION---------------------- chemotherapeutic agents for cancer. Pain on injection was observed more Reconstitute vial contents and dilute before use. (2.3) frequently in patients receiving ZINECARD versus placebo. (6.1) Administer ZINECARD by intravenous infusion over 15 minutes. DO NOT ADMINISTER VIA AN INTRAVENOUS PUSH. (2.1, 2.3) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at The recommended dosage ratio of ZINECARD to doxorubicin is 10:1 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

Metabolic Carbonyl Reduction of Anthracyclines — Role in Cardiotoxicity and Cancer Resistance
Invest New Drugs DOI 10.1007/s10637-017-0443-2 REVIEW Metabolic carbonyl reduction of anthracyclines — role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents Kamil Piska1 & Paulina Koczurkiewicz1 & Adam Bucki 2 & Katarzyna Wójcik-Pszczoła1 & Marcin Kołaczkowski2 & Elżbieta Pękala1 Received: 23 November 2016 /Accepted: 17 February 2017 # The Author(s) 2017. This article is published with open access at Springerlink.com Summary Anthracycline antibiotics (ANT), such as doxoru- monoHER, curcumin, (−)-epigallocatechin gallate, resvera- bicin or daunorubicin, are a class of anticancer drugs that are trol, berberine or pixantrone, and their modulating effect on widely used in oncology. Although highly effective in cancer the activity of ANT is characterized and discussed as potential therapy, their usefulness is greatly limited by their mechanism of action for novel therapeutics in cancer cardiotoxicity. Possible mechanisms of ANT cardiotoxicity treatment. include their conversion to secondary alcohol metabolites (i.e. doxorubicinol, daunorubicinol) catalyzed by carbonyl re- Keywords Anthracyclines . Cardiotoxicity . Resistance . ductases (CBR) and aldo-keto reductases (AKR). These me- Pharmacokinetics . Drug metabolism . Anticancer agents tabolites are suspected to be more cardiotoxic than their parent compounds. Moreover, overexpression of ANT-reducing en- zymes (CBR and AKR) are found in many ANT-resistant Introduction cancers. The secondary metabolites show decreased cytotoxic properties and are more susceptible to ABC-mediated efflux Anthracyclines (ANT) are a class of cell-cycle non-specific than their parent compounds; thus, metabolite formation is anticancer antibiotics that were first isolated from the considered one of the mechanisms of cancer resistance. Streptomyces genus in the early 1960s. -
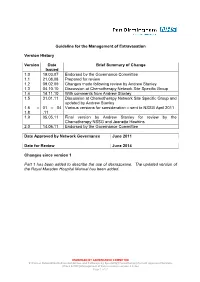
Guideline for the Management of Extravasation
Guideline for the Management of Extravasation Version History Version Date Brief Summary of Change Issued 1.0 19.03.07 Endorsed by the Governance Committee 1.1 21.08.08 Prepared for review 1.2 09.02.09 Changes made following review by Andrew Stanley 1.3 04.10.10 Discussion at Chemotherapy Network Site Specific Group 1.4 14.11.10 With comments from Andrew Stanley 1.5 31.01.11 Discussion at Chemotherapy Network Site Specific Group and updated by Andrew Stanley 1.6 – 01 – 04 Various versions for consideration – sent to NSSG April 2011 1.8 .11 1.9 05.05.11 Final version by Andrew Stanley for review by the Chemotherapy NSSG and Jeanette Hawkins 2.0 14.06.11 Endorsed by the Governance Committee Date Approved by Network Governance June 2011 Date for Review June 2014 Changes since version 1 Part 1 has been added to describe the use of dexrazoxane. The updated version of the Royal Marsden Hospital Manual has been added. ENDORSED BY GOVERNANCE COMMITTEE S:\Cancer Network\Guidelines\Guidelines and Pathways by Speciality\Chemotherapy\Current Approved Versions (Word & PDF)\Management of Extravasation version 2.0.doc Page 1 of 21 1 Scope of the Guideline This guidance has been produced to support the following: The prevention of the extravasation of intravenous anti-cancer drugs. The early detection of the extravasation of intravenous anti-cancer drugs. The treatment of the extravasation of intravenous anti-cancer drugs. 2 Guideline Statement Statement 2 The Network Site Specific Group has agreed to adopt the Royal Marsden Hospital Manual of Clinical Nursing Procedures 7th Edition; Blackwell Publishing (2008), chapter on extravasation, with the addition of a section on dexrazoxane.