Spatial Patch Occupancy Patterns of the Lower Keys Marsh Rabbit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -
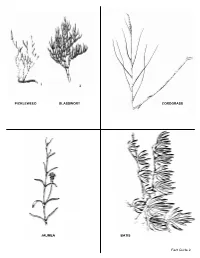
Plant Field Guide
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

Eastern Cottontail Rabbits (Sylvilagus Floridanus)
Publication WSFNR-20-58A July 2020 Managing Wildlife Damage: Eastern Cottontail Rabbits (Sylvilagus floridanus) Joseph Brown, UGA Warnell School of Forestry & Natural Resources Michael T. Mengak, UGA Warnell School of Forestry & Natural Resources INTRODUCTION Eastern cottontail rabbits (Sylvilagus floridanus) are found across the eastern United States, southern Canada, eastern Mexico, Central America and portions of South America (Figure 1). Cottontail rabbits are a sought-after game species by small game hunters. Many people enjoy seeing them around their yards and neighborhoods. Rabbits are well accepted by humans when their numbers are managed correctly. However, they can inflict damage across a range of habitats from rural farms to suburban lawns. They often damage flowers, vegetable gardens, and landscape trees and shrubs. TAXONOMY Class Mammalia Order Lagomorpha Family Leporidae Genus - Sylvilagus Species – floridanus Common Name - Eastern cottontail rabbit There are 11 genera and 54 species in Leporidae which includes hares and rabbits. Six species of cottontail rabbits are found in the genus Sylvilagus. The scientific name, Sylvilagus floridanus, means forest hare of Florida. Figure 1: Current eastern cottontail STATUS distribution across North and Central America. In Georgia, we have 4 native species of rabbits. The swamp rabbit (Sylvilagus aquaticus), Appalachian cottontail (S. obscurus), marsh rabbit (S. palustris), and eastern cottontail (S. floridanus). The eastern cottontail (Figure 2) is the most abundant and ranges across the entire state. The Appalachian cottontail is the rarest rabbit and inhabits the start of the Appalachian mountain chain in North Georgia. It is on the Georgia Protected Wildlife list. The swamp rabbit is the largest of the four and inhabits swamps in the Piedmont region of Georgia. -

Population Structure of the Lower Keys Marsh Rabbit As Determined by Mitochondrial DNA Analysis
Management and Conservation Note Population Structure of the Lower Keys Marsh Rabbit as Determined by Mitochondrial DNA Analysis AMANDA L. CROUSE, College of Veterinary Medicine, Texas A&M University, College Station, TX 77843-4461, USA RODNEY L. HONEYCUTT, Natural Science Division, Pepperdine University, Malibu, CA 90263-4321, USA ROBERT A. MCCLEERY,1 Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258, USA CRAIG A. FAULHABER, Department of Wildland Resources, Utah State University, Logan, UT 84322-5230, USA NEIL D. PERRY, Utah Division of Wildlife Resources, Cedar City, UT 84270-0606, USA ROEL R. LOPEZ, Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258, USA ABSTRACT We used nucleotide sequence data from a mitochondrial DNA fragment to characterize variation within the endangered Lower Keys marsh rabbit (Sylvilagus palustris hefneri). We observed 5 unique mitochondrial haplotypes across different sampling sites in the Lower Florida Keys, USA. Based on the frequency of these haplotypes at different geographic locations and relationships among haplotypes, we observed 2 distinct clades or groups of sampling sites (western and eastern clades). These 2 groups showed low levels of gene flow. Regardless of their origin, marsh rabbits from the Lower Florida Keys can be separated into 2 genetically distinct management units, which should be considered prior to implementation of translocations as a means of offsetting recent population declines. (JOURNAL OF WILDLIFE MANAGEMENT 73(3):362–367; 2009) DOI: 10.2193/2007-207 KEY WORDS Florida Keys, genetic, marsh rabbit, mitochondrial DNA, population structure, Sylvilagus palustris hefneri. The Lower Keys marsh rabbit (Sylvilagus palustris hefneri)is (Forys and Humphrey 1999b). -

Informes Individuales IUCN 2018.Indd
IUCN SSC Lagomorph Specialist Group 2018 Report Andrew Smith Hayley Lanier Co-Chairs Mission statement Targets for the 2017-2020 quadrennium Andrew Smith (1) To promote the conservation and effective Assess (2) Hayley Lanier sustainable management of all species of Red List: (1) improve knowledge and assess- lagomorph through science, education and ment of lagomorph systematics, (2) complete Red List Authority Coordinator advocacy. all Red List reassessments of all lagomorph Charlotte Johnston (1) species. Projected impact for the 2017-2020 Research activities: (1) improve knowledge of Location/Affiliation quadrennium Brachylagus idahoensis; (2) examine popula- (1) School of Life Sciences, Arizona State The Lagomorph Specialist Group (LSG) is tion trends of all lagomorphs in the western University, Tempe, Arizona, US “middle-sized” – not a single species, nor United States; (3) improve knowledge of Lepus (2) Sam Noble Museum, University of Oklahoma, composed of hundreds of species. We have callotis; (4) improve knowledge of Lepus fagani, Norman, Oklahoma, US slightly less than 100 species in our brief. L. habessinicus, and L. starcki in Ethiopia; However, these are distributed around the (5) improve knowledge of Lepus flavigularis; Number of members globe, and there are few similarities among (6) improve knowledge of all Chinese Lepus; 73 any of our many forms that are Red List clas- (7) improve knowledge of Nesolagus netscheri; sified as Threatened. Thus, we do not have a (8) improve knowledge of Nesolagus timminsi; Social networks single programme or a single thrust; there is no (9) improve knowledge of Ochotona iliensis; Website: one-size-fits-all to our approach. LSG members (10) improve surveys of poorly-studied www.lagomorphspecialistgroup.org largely work independently in their region, and Ochotona in China; (11) understand the role the Co-Chairs serve more as a nerve centre. -

Rare Plants of Louisiana
Rare Plants of Louisiana Agalinis filicaulis - purple false-foxglove Figwort Family (Scrophulariaceae) Rarity Rank: S2/G3G4 Range: AL, FL, LA, MS Recognition: Photo by John Hays • Short annual, 10 to 50 cm tall, with stems finely wiry, spindly • Stems simple to few-branched • Leaves opposite, scale-like, about 1mm long, barely perceptible to the unaided eye • Flowers few in number, mostly born singly or in pairs from the highest node of a branchlet • Pedicels filiform, 5 to 10 mm long, subtending bracts minute • Calyx 2 mm long, lobes short-deltoid, with broad shallow sinuses between lobes • Corolla lavender-pink, without lines or spots within, 10 to 13 mm long, exterior glabrous • Capsule globe-like, nearly half exerted from calyx Flowering Time: September to November Light Requirement: Full sun to partial shade Wetland Indicator Status: FAC – similar likelihood of occurring in both wetlands and non-wetlands Habitat: Wet longleaf pine flatwoods savannahs and hillside seepage bogs. Threats: • Conversion of habitat to pine plantations (bedding, dense tree spacing, etc.) • Residential and commercial development • Fire exclusion, allowing invasion of habitat by woody species • Hydrologic alteration directly (e.g. ditching) and indirectly (fire suppression allowing higher tree density and more large-diameter trees) Beneficial Management Practices: • Thinning (during very dry periods), targeting off-site species such as loblolly and slash pines for removal • Prescribed burning, establishing a regime consisting of mostly growing season (May-June) burns Rare Plants of Louisiana LA River Basins: Pearl, Pontchartrain, Mermentau, Calcasieu, Sabine Side view of flower. Photo by John Hays References: Godfrey, R. K. and J. W. Wooten. -

Vascular Plants of Pu'uhonua 0 Hiinaunau National
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ScholarSpace at University of Hawai'i at Manoa Technical Report 105 Vascular Plants of Pu'uhonua 0 Hiinaunau National Historical Park Technical Report 106 Birds of Pu'uhonua 0 Hiinaunau National Historical Park COOPERATIVE NATIONAL PARK RESOURCES STUDIES UNIT UNIVERSITY OF HAWAI'I AT MANOA Department of Botany 3 190 Maile Way Honolulu, Hawai'i 96822 (808) 956-8218 Clifford W. Smith, Unit Director Technical Report 105 VASCULAR PLANTS OF PU'UHONUA 0 HONAUNAU NATIONAL HISTORICAL PARK Linda W. Pratt and Lyman L. Abbott National Biological Service Pacific Islands Science Center Hawaii National Park Field Station P. 0.Box 52 Hawaii National Park, HI 967 18 University of Hawai'i at Manoa National Park Service Cooperative Agreement CA8002-2-9004 May 1996 TABLE OF CONTENTS Page . LIST OF FIGURES ............................................. 11 ABSTRACT .................................................. 1 ACKNOWLEDGMENTS .........................................2 INTRODUCTION ..............................................2 THESTUDYAREA ............................................3 Climate ................................................ 3 Geology and Soils ......................................... 3 Vegetation ..............................................5 METHODS ...................................................5 RESULTS AND DISCUSSION .....................................7 Plant Species Composition ...................................7 Additions to the -

Appendix Lagomorph Species: Geographical Distribution and Conservation Status
Appendix Lagomorph Species: Geographical Distribution and Conservation Status PAULO C. ALVES1* AND KLAUS HACKLÄNDER2 Lagomorph taxonomy is traditionally controversy, and as a consequence the number of species varies according to different publications. Although this can be due to the conservative characteristic of some morphological and genetic traits, like general shape and number of chromosomes, the scarce knowledge on several species is probably the main reason for this controversy. Also, some species have been discovered only recently, and from others we miss any information since they have been first described (mainly in pikas). We struggled with this difficulty during the work on this book, and decide to include a list of lagomorph species (Table 1). As a reference, we used the recent list published by Hoffmann and Smith (2005) in the “Mammals of the world” (Wilson and Reeder, 2005). However, to make an updated list, we include some significant published data (Friedmann and Daly 2004) and the contribu- tions and comments of some lagomorph specialist, namely Andrew Smith, John Litvaitis, Terrence Robinson, Andrew Smith, Franz Suchentrunk, and from the Mexican lagomorph association, AMCELA. We also include sum- mary information about the geographical range of all species and the current IUCN conservation status. Inevitably, this list still contains some incorrect information. However, a permanently updated lagomorph list will be pro- vided via the World Lagomorph Society (www.worldlagomorphsociety.org). 1 CIBIO, Centro de Investigaça˜o em Biodiversidade e Recursos Genéticos and Faculdade de Ciˆencias, Universidade do Porto, Campus Agrário de Vaira˜o 4485-661 – Vaira˜o, Portugal 2 Institute of Wildlife Biology and Game Management, University of Natural Resources and Applied Life Sciences, Gregor-Mendel-Str. -

The Coastal Habitats and Vegetation of the Kimberley Region
Journal of the Royal Society of Western Australia, 94: 197–206, 2011 The coastal habitats and vegetation of the Kimberley region I D Cresswell 1, P Bridgewater 2 & V Semeniuk 3 1 CSIRO Wealth from Oceans Flagship, GPO Box 1538, Hobart, Tasmania, 7001 2 The Wharfside, Station Place, Peel, Isle of Man, British Isles 3 V & C Semeniuk Research Group, 21 Glenmere Rd., Warwick, W.A., 6024 Manuscript received March 2011; accepted April 2011 Abstract Incorporating the areas of the rocky Kimberley Coast, flanked by the deltaic gulfs of Cambridge Gulf and King Sound, as well as the Dampier Peninsula, the Kimberley region host a complicated coastal zone with a plethora of coastal habitats. The smallest scale of habitat includes rocky cliff, scree slopes, gravelly/bouldery shore, sandy beaches, spits, dunes, tidal mud flats, alluvial fans, and the contact between some of these habitats and freshwater. The main vegetation units include mangroves, shrubby chenopods (which include succulent halophytic shrubs), saline marsh, sedgelands, rushlands, dune scrub, dune grasslands, and teatree thickets. The spatially and temporally variable landscape, sediments/soils, and hydrochemistry expressed at the coast mean that the coastal vegetation habitats are the most complex habitats in the Kimberley region. This review found that to date these have not been fully explored or described botanically. Keywords: Kimberley Coast, coast, coastal habitats, mangrove, saltmarsh, mangal, chenopod Introduction formations, soils, water availability, and climate; in the coastal zone vegetation distribution (and its component Perhaps the first general survey of coastal ecosystems flora) responds to a wider variety of environmental in Western Australia was Sauer (1965), which dealt, inter factors including physiography, groundwater alia, with the Kimberley coast. -

HUNTIA a Journal of Botanical History
HUNTIA A Journal of Botanical History VolUme 11 NUmBer 1 2000 Hunt Institute for Botanical Documentation Carnegie mellon University Pittsburgh Huntia publishes articles on all aspects of the his- tory of botany and is published irregularly in one or more numbers per volume of approximately 200 pages by the Hunt Institute for Botanical Documentation, Carnegie mellon University, Pittsburgh, Pennsylvania 15213-3890. editor Scarlett T. Townsend Book reviews and Announcements editor Charlotte A. Tancin Associate editors Gavin D. r. Bridson T. D. Jacobsen Angela l. Todd Frederick H. Utech James J. White layout Lugene B. Bruno external contributions to Huntia are welcome. Please request our “Guidelines for Contributors” before submitting manuscripts for consideration. editorial correspondence should be directed to the editor. Books for announcement or review should be sent to the Book reviews and Announcements editor. Page charge is $50.00. The charges for up to five pages per year are waived for Hunt Institute Associates, who also may elect to receive Huntia as a benefit of membership; please contact the Institute for more information. Subscription rate is $60.00 per volume. orders for subscriptions and back issues should be sent to the Institute. Printed and bound by Allen Press, Inc., lawrence, Kansas. Copyright © 2000 by the Hunt Institute for Botanical Documentation All rights reserved ISSN 0073-4071 Huntia 11(1) 2000 Jamaican plant genera named by Patrick Browne (ca. 1720–1790): A checklist with an attempt at an etymology P. H. Oswald and E. Charles Nelson Abstract Patrick Browne’s generic names for Jamaican native them. Most meanings are taken from Liddell plants, published during 1756, are listed and their and Scott (1940) and Lewis and Short (1879). -

Adopt-A-Pond Notebook
CONGRATULATIONS! This Is Your Group’s Adopt-A-Pond Notebook Let this notebook help your pond group take care of your pond environment, and improve water quality through stormwater pollution prevention. Use it to organize and record your Adopt-A-Pond activities. Have a pond question? Look in your notebook for the answer! The Adopt-A-Pond Notebook: Use it to learn more about your pond environment. Adopt-A-Pond Notebook Table of Contents: Chapter 1 Program Information Chapter 2 Forms Chapter 3 Management Chapter 4 Prevention Chapter 5 Wildlife and Habitat The Adopt-A-Pond Notebook: Use it to learn more about your pond environment. "Adopt-A-Pond" is a volunteer-based program sponsored by Hillsborough County and The Southwest Florida Water Management District. 2420 N Falkenburg Rd. Tampa, Florida 33619 Phone (813) 744-5671 FAX (813) 744-5674 Chapter 1 Program Information 9 Statement of Adopt-A-Pond Goals 9 Adopt-A-Pond Committee Member List 9 Adopt-A-Pond Plant Species List 9 Keep Adopt-A-Pond Agreement Here 9 Recommended Book List The Adopt-A-Pond Notebook: Use it to learn more about your pond environment. Adopt-A-Pond Program Goals • Stormwater Pollution Prevention • Cleaner Water & Better Pond Environments • Beautiful Ponds • Increased Wildlife Habitat • Fewer Nuisance Plants • Participate in Adopt-A-Pond Activities • Receive "On Our Pond" Newsletter Adopt-A-Pond Program Activities Neighborhood Education Meeting Neighborhood Pond Planting Storm Drain Marking Neighborhood Pond Work Days Pond Work Day Reports Pond Walks (March through October) Best Maintained Pond Contest Annual Pond Seminar Water Quality Testing Kits The Adopt-A-Pond Notebook: Use it to learn more about your pond environment. -

The Biology of Limonium Carolinianum (Walt.) Britt. (Plumbaginaceae) in Coastal Wetlands
International Biology Review The Biology of Limonium carolinianum (Walt.) Britt. (Plumbaginaceae) in Coastal Wetlands Richard Stalter*, Robert I. Lonard**, and Frank W. Judd** *Department of Biological ABSTRACT Sciences, St. John’s University, 8000 Utopia Limonium carolinianum (Walt.) Britt. is a New World humid Parkway, Queens, NY 11439 continental, temperate, subtropical, and tropical maritime taxon. It is a long-lived perennial that is found in wet sand **Department of Biology, and clay mineral soils. This species is a salt tolerant halophyte The University of Texas – Rio where salinity values approach 30 parts per thousand. Grande Valley, Edinburg, TX Reproduction is primarily vegetative. However, seed 78539 production is estimated at 1,000 to 10,000 per individual plant. Also known as sea lavender, it has been suggested as a Corresponding Author: species that can be used for re-vegetation efforts. Dr. Richard Stalter Inflorescences of this species are often harvested for dried ([email protected]) floral arrangements. Keywords: Sea lavender, morphology, habitats, communities, population ecology, physiological ecology, economic values. Copyright 2016 KEI Journals. All rights reserved 1 International Biology Review INTRODUCTION statice. The following taxonomic description has been derived from Correll and Correll The genus Limonium (Plumbaginaceae) 1972; Flora of North America Editorial includes about 300 to 350 species, and is Committee 2005; Hamilton 1997; Lehman primarily represented in maritime or in arid 2013; Luteyn 1976, 1990; and Radford et al. environments in the northern hemisphere 1968. (Correll and Correll 1972; Mabberley 1997). Eight species have been reported in North Limonium carolinianum has a woody taproot America including four species that have typical of dicots that extend up to 30.5 cm escaped from cultivation in California (Flora below the soil surface (R.