Estimating Diet and Food Selectivity of the Lower Keys Marsh Rabbit Using Stable Isotope Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Conocarpus Erectus
Conocarpus erectus (Button Mangrove, Green Buttonwood) Button mangrove is a broadleaf evergreen trees which can withstand drought, salt, heat and high winds.The fruit looks like a dried raspberry or a pine cone. Its flaky brown bark is very attractive. Throughout the year, greenish-white and purple flowers are produced, but they are not noticeable. Due to the high tolerance of heat and drought it is used a lot in hot and arid climate as hedge, street tree or windbreak. Landscape Information French Name: Chêne Guadeloupe ﺩﻣﺲ ﻗﺎﺋﻢ :Arabic Name Pronounciation: kawn-oh-KAR-pus ee-RECK- tus Plant Type: Tree Origin: Florida and the West Indies Heat Zones: 9, 10, 11, 12, 14, 15, 16 Hardiness Zones: 10, 11, 12, 13 Uses: Screen, Hedge, Bonsai, Specimen, Container, Shade, Windbreak, Pollution Tolerant / Urban, Reclamation Size/Shape Growth Rate: Moderate Plant Image Tree Shape: Spreading, Vase Canopy Symmetry: Symmetrical Canopy Density: Medium Canopy Texture: Fine Height at Maturity: 8 to 15 m Spread at Maturity: 8 to 10 meters Conocarpus erectus (Button Mangrove, Green Buttonwood) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Pinnate Leaf Persistance: Evergreen Leaf Type: Simple Leaf Blade: 5 - 10 cm Leaf Shape: Lanceolate Leaf Margins: Entire Leaf Textures: Glossy, Fine Leaf Scent: No Fragance Color(growing season): Green Color(changing season): Green Flower Image Flower Flower Showiness: False Flower Color: Green, White Seasons: Year Round Trunk Trunk Susceptibility to Breakage: Generally resists breakage Number of -

Overview of Rabbit Hemorrhagic Disease
Overview of Rabbit Hemorrhagic Disease Dr. Amber Itle Dr. Susan Kerr [email protected] [email protected] 360-961-4129 360-789-7664 Previous isolated U.S. cases in owned domestic rabbits 2000 (IA) The 2001 2019 (UT, IL, NY) Washington 2005 (IN) State Outbreak2008, 2010, 2018 (OH, PA) Rabbits at Risk Tame/owned & feral domestic rabbits (European rabbits, Oryctolagus cuniculus) Rabbits (Probably) Not at Risk WILD RABBITS Snowshoe hare (Lepus americanus) European brown hare (Lepus europaeus) Black-tailed jackrabbit (Lepus californicus) White-tailed jackrabbit (Lepus townsendi) Volcano rabbit (Romerolagus diazzi) Pygmy rabbit (Brachylagus idahoensis) Eastern cottontail (Sylvilagus floridanus) Nuttall's or mountain cottontail (Sylvilagus nuttallii) World Animal Health Information Database OIE World Organisation for Animal Health www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/WI We’re famous! Timeline of RHD Cases in WA, 2019 • Index case: Single owned domestic rabbit, Orcas Island July 9 • RISK FACTOR: Rodents, farm hygiene • Case 2: Feral domestic rabbits die off, Orcas Island July 11 July • Suspect cases: Feral domestic rabbitJuly die-Dec.,-off, Lopez 2019:Island 18-24 Owned and feral • Case 3: 14/25 domestic owned meat rabbits die, Orcas Island July 24 • RISK FACTOR: Vegetationdomestic cut for bedding andrabbits forage tested • Case 4: 2/5 domestic ownednegative rabbits die, feral King, domestic Skagit, die-off San Juan Aug 2 • RISK FACTOR: Direct contactPierce, with ferals andthrough Clallam cages Co. • Case 5: 11/33 domestic -

Conocarpus Erectus" Plant As Biomonitoring of Soil and Air Pollution in Ahwaz Region
Middle-East Journal of Scientific Research 13 (10): 1319-1324, 2013 ISSN 1990-9233 © IDOSI Publications, 2013 DOI: 10.5829/idosi.mejsr.2013.13.10.1182 Evaluation of "Conocarpus erectus" Plant as Biomonitoring of Soil and Air Pollution in Ahwaz Region 12Ali Gholami, Amir Hossein Davami, 3Ebrahim Panahpour and 4Hossein Amini 1,3Department of Soil Science, Science and Research Branch, Islamic Azad University, Khouzestan, Iran 2Department of Environmental Management, Science and Research Branch, Islamic Azad University, Khouzestan, Iran 4Department of Soil Science, Islamic Azad University, Khorasgan Branch, Isfahan, Iran Abstract: Effects of soil and atmosphere pollution on some heavy metals (Fe, Zn, Pb, Cu, Mn and Cd) concentration in Button-tree (Conocarpus erectus) leaves were studied in the city of Ahwaz (Khouzestan, Iran). Samples were collected from four sampling sites representing area of high traffic density, area future away from traffic and Industrial area. Samples were collected in two stages (May and October) in 2011 for chemical analysis. Samples from village near the city also analyzed for comparison. Based on the results, the stages of leaf sampling did not showed any significant effect on the concentration of the measured heavy metals in leaf samples. Chemical analysis of soil samples at depth of 0-10cm showed that concentration of most of these elements was lower than the maximum recommended levels. Concentrations of measured heavy metals in washed leaves were lower than those of unwashed leaves of Conocarpus and different was significant. In spite of that, there was no significant correlation between the concentrations of heavy metals in washed leaves and soil samples. -
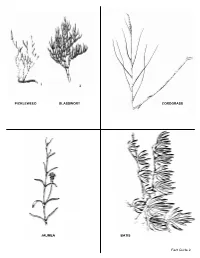
Plant Field Guide
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

Eastern Cottontail Rabbits (Sylvilagus Floridanus)
Publication WSFNR-20-58A July 2020 Managing Wildlife Damage: Eastern Cottontail Rabbits (Sylvilagus floridanus) Joseph Brown, UGA Warnell School of Forestry & Natural Resources Michael T. Mengak, UGA Warnell School of Forestry & Natural Resources INTRODUCTION Eastern cottontail rabbits (Sylvilagus floridanus) are found across the eastern United States, southern Canada, eastern Mexico, Central America and portions of South America (Figure 1). Cottontail rabbits are a sought-after game species by small game hunters. Many people enjoy seeing them around their yards and neighborhoods. Rabbits are well accepted by humans when their numbers are managed correctly. However, they can inflict damage across a range of habitats from rural farms to suburban lawns. They often damage flowers, vegetable gardens, and landscape trees and shrubs. TAXONOMY Class Mammalia Order Lagomorpha Family Leporidae Genus - Sylvilagus Species – floridanus Common Name - Eastern cottontail rabbit There are 11 genera and 54 species in Leporidae which includes hares and rabbits. Six species of cottontail rabbits are found in the genus Sylvilagus. The scientific name, Sylvilagus floridanus, means forest hare of Florida. Figure 1: Current eastern cottontail STATUS distribution across North and Central America. In Georgia, we have 4 native species of rabbits. The swamp rabbit (Sylvilagus aquaticus), Appalachian cottontail (S. obscurus), marsh rabbit (S. palustris), and eastern cottontail (S. floridanus). The eastern cottontail (Figure 2) is the most abundant and ranges across the entire state. The Appalachian cottontail is the rarest rabbit and inhabits the start of the Appalachian mountain chain in North Georgia. It is on the Georgia Protected Wildlife list. The swamp rabbit is the largest of the four and inhabits swamps in the Piedmont region of Georgia. -

Chromosome Numbers in Compositae, XII: Heliantheae
SMITHSONIAN CONTRIBUTIONS TO BOTANY 0 NCTMBER 52 Chromosome Numbers in Compositae, XII: Heliantheae Harold Robinson, A. Michael Powell, Robert M. King, andJames F. Weedin SMITHSONIAN INSTITUTION PRESS City of Washington 1981 ABSTRACT Robinson, Harold, A. Michael Powell, Robert M. King, and James F. Weedin. Chromosome Numbers in Compositae, XII: Heliantheae. Smithsonian Contri- butions to Botany, number 52, 28 pages, 3 tables, 1981.-Chromosome reports are provided for 145 populations, including first reports for 33 species and three genera, Garcilassa, Riencourtia, and Helianthopsis. Chromosome numbers are arranged according to Robinson’s recently broadened concept of the Heliantheae, with citations for 212 of the ca. 265 genera and 32 of the 35 subtribes. Diverse elements, including the Ambrosieae, typical Heliantheae, most Helenieae, the Tegeteae, and genera such as Arnica from the Senecioneae, are seen to share a specialized cytological history involving polyploid ancestry. The authors disagree with one another regarding the point at which such polyploidy occurred and on whether subtribes lacking higher numbers, such as the Galinsoginae, share the polyploid ancestry. Numerous examples of aneuploid decrease, secondary polyploidy, and some secondary aneuploid decreases are cited. The Marshalliinae are considered remote from other subtribes and close to the Inuleae. Evidence from related tribes favors an ultimate base of X = 10 for the Heliantheae and at least the subfamily As teroideae. OFFICIALPUBLICATION DATE is handstamped in a limited number of initial copies and is recorded in the Institution’s annual report, Smithsonian Year. SERIESCOVER DESIGN: Leaf clearing from the katsura tree Cercidiphyllumjaponicum Siebold and Zuccarini. Library of Congress Cataloging in Publication Data Main entry under title: Chromosome numbers in Compositae, XII. -

TAXON:Conocarpus Erectus L. SCORE:5.0 RATING:Evaluate
TAXON: Conocarpus erectus L. SCORE: 5.0 RATING: Evaluate Taxon: Conocarpus erectus L. Family: Combretaceae Common Name(s): button mangrove Synonym(s): Conocarpus acutifolius Willd. ex Schult. buttonwood Conocarpus procumbens L. Sea mulberry Assessor: Chuck Chimera Status: Assessor Approved End Date: 30 Jul 2018 WRA Score: 5.0 Designation: EVALUATE Rating: Evaluate Keywords: Tropical Tree, Naturalized, Coastal, Pure Stands, Water-Dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 n outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) n 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals 405 Toxic to animals y=1, n=0 n 406 Host for recognized pests and pathogens 407 Causes allergies or is otherwise toxic to humans y=1, n=0 n 408 Creates a fire hazard in natural ecosystems y=1, n=0 n 409 Is a shade tolerant plant at some stage of its life cycle y=1, n=0 n Creation Date: 30 Jul 2018 (Conocarpus erectus L.) Page 1 of 17 TAXON: Conocarpus erectus L. -

A Caenorhabditis Elegans Model for Discovery of Novel Anti-Infectives
fmicb-07-01956 November 30, 2016 Time: 12:40 # 1 REVIEW published: 02 December 2016 doi: 10.3389/fmicb.2016.01956 Beyond Traditional Antimicrobials: A Caenorhabditis elegans Model for Discovery of Novel Anti-infectives Cin Kong†, Su-Anne Eng, Mei-Perng Lim and Sheila Nathan* School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia The spread of antibiotic resistance amongst bacterial pathogens has led to an urgent need for new antimicrobial compounds with novel modes of action that minimize the potential for drug resistance. To date, the development of new antimicrobial drugs is still lagging far behind the rising demand, partly owing to the absence of an effective screening platform. Over the last decade, the nematode Caenorhabditis elegans Edited by: Luis Cláudio Nascimento Da Silva, has been incorporated as a whole animal screening platform for antimicrobials. This CEUMA University, Brazil development is taking advantage of the vast knowledge on worm physiology and how it Reviewed by: interacts with bacterial and fungal pathogens. In addition to allowing for in vivo selection Osmar Nascimento Silva, of compounds with promising anti-microbial properties, the whole animal C. elegans Universidade Católica Dom Bosco, Brazil screening system has also permitted the discovery of novel compounds targeting Francesco Imperi, infection processes that only manifest during the course of pathogen infection of the Sapienza University of Rome, Italy host. Another advantage of using C. elegans in the search for new antimicrobials is that *Correspondence: Sheila Nathan the worm itself is a source of potential antimicrobial effectors which constitute part of its [email protected] immune defense response to thwart infections. -

The Volcano Rabbit—A Shrinking Distribution and a Threatened Habitat
The volcano rabbit— a shrinking distribution and a threatened habitat J. Hoth, A. Velazquez, F. J. Romero, L. Leon, M. Aranda and D. J. Bell The first systematic search for the volcano rabbit, a Mexican endemic, found no evidence of the species outside the central region of the transverse neovolcanic axis around Mexico City, and reveals that the rabbit has now disappeared from areas of this central zone where it has been reported in the recent past. It depends for its survival on the pine forest—bunch grass ecosystem, and this is being destroyed by conversion to agriculture, over-grazing, fire-burns, poor forestry management and encroaching property developments. The authors make recommendations for the immediate protection of the remaining areas of core habitat and for further research. The volcano rabbit, or 'zacatuche', Romerolagus the uncontrolled burning of 'zacaton' grasses to diazi is believed to be amongst the most primitive promote new growth of grazing pasture for cattle of the living species of rabbits and hares (O. and sheep, plus the exploitation of zacaton for the Lagomorpha; Family: Leporidae) (De Poorter manufacture of household brushes (Thornback and Van der Loo, 1981; Corbet, 1983; Barrera, and Jenkins, 1982; Granados, 1981). The aims 1966). of the present study were, firstly, to characterize the habitat of known populations of R. diazi in Prior to the present study no systematic search for terms of altitude, temperature, climate and this Mexican endemic had ever been carried out, dominant vegetation types, and secondly, to yet its distribution is repeatedly described as being conduct an extensive search for the species in restricted to scattered areas of sub-alpine pine- these and other areas of equivalent habitat across 'zacaton' (bunch grass) and alder-'zacaton' the entire transverse neovolcanic axis. -

Population Structure of the Lower Keys Marsh Rabbit As Determined by Mitochondrial DNA Analysis
Management and Conservation Note Population Structure of the Lower Keys Marsh Rabbit as Determined by Mitochondrial DNA Analysis AMANDA L. CROUSE, College of Veterinary Medicine, Texas A&M University, College Station, TX 77843-4461, USA RODNEY L. HONEYCUTT, Natural Science Division, Pepperdine University, Malibu, CA 90263-4321, USA ROBERT A. MCCLEERY,1 Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258, USA CRAIG A. FAULHABER, Department of Wildland Resources, Utah State University, Logan, UT 84322-5230, USA NEIL D. PERRY, Utah Division of Wildlife Resources, Cedar City, UT 84270-0606, USA ROEL R. LOPEZ, Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258, USA ABSTRACT We used nucleotide sequence data from a mitochondrial DNA fragment to characterize variation within the endangered Lower Keys marsh rabbit (Sylvilagus palustris hefneri). We observed 5 unique mitochondrial haplotypes across different sampling sites in the Lower Florida Keys, USA. Based on the frequency of these haplotypes at different geographic locations and relationships among haplotypes, we observed 2 distinct clades or groups of sampling sites (western and eastern clades). These 2 groups showed low levels of gene flow. Regardless of their origin, marsh rabbits from the Lower Florida Keys can be separated into 2 genetically distinct management units, which should be considered prior to implementation of translocations as a means of offsetting recent population declines. (JOURNAL OF WILDLIFE MANAGEMENT 73(3):362–367; 2009) DOI: 10.2193/2007-207 KEY WORDS Florida Keys, genetic, marsh rabbit, mitochondrial DNA, population structure, Sylvilagus palustris hefneri. The Lower Keys marsh rabbit (Sylvilagus palustris hefneri)is (Forys and Humphrey 1999b). -

Borrichia Frutescens Sea Oxeye1 Edward F
FPS69 Borrichia frutescens Sea Oxeye1 Edward F. Gilman2 Introduction USDA hardiness zones: 10 through 11 (Fig. 2) Planting month for zone 10 and 11: year round The sea oxeye daisy is a true beach plant and may be used Origin: native to Florida in the landscape as a flowering hedge or ground cover (Fig. Uses: mass planting; ground cover; attracts butterflies 1). It spreads by rhizomes and attains a height of 2 to 4 feet. Availability: somewhat available, may have to go out of the The foliage of this plant is fleshy and gray-green in color. region to find the plant The flowering heads of Borrichia frutescens have yellow rays with brownish-yellow disc flowers, and these flowers attract many types of butterflies. Each flower is subtended by a hard, erect, sharp bract. The fruits are small, inconspicuous, four-sided achenes. Figure 2. Shaded area represents potential planting range. Description Height: 2 to 3 feet Figure 1. Sea oxeye. Spread: 2 to 3 feet Plant habit: upright General Information Plant density: moderate Growth rate: slow Scientific name: Borrichia frutescens Texture: medium Pronunciation: bor-RICK-ee-uh froo-TESS-enz Common name(s): sea oxeye Foliage Family: Compositae Plant type: ground cover Leaf arrangement: opposite/subopposite 1. This document is FPS69, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date October 1999. Reviewed February 2014. Visit the EDIS website at http://edis.ifas.ufl.edu. 2. Edward F. Gilman, professor, Environmental Horticulture Department, UF/IFAS Extension, Gainesville, FL 32611. The Institute of Food and Agricultural Sciences (IFAS) is an Equal Opportunity Institution authorized to provide research, educational information and other services only to individuals and institutions that function with non-discrimination with respect to race, creed, color, religion, age, disability, sex, sexual orientation, marital status, national origin, political opinions or affiliations.