Flow Field Effect on the Performance of Direct Formic Acid Membraneless Fuel Cells: a Numerical Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of Membraneless Mixed-Reactant Microfluidic Fuel Cells: Electrocatalysis and Evolution Through Numerical Simulation
Université du Québec Institut National de la Recherche Scientifique Centre Énergie, Matériaux et Télécommunications DEVELOPMENT OF MEMBRANELESS MIXED-REACTANT MICROFLUIDIC FUEL CELLS: ELECTROCATALYSIS AND EVOLUTION THROUGH NUMERICAL SIMULATION Par Juan Carlos Abrego Martínez Thèse présentée pour l’obtention du grade de Philosophiae Doctor (Ph.D.) en sciences de l’énergie et des matériaux Jury d’évaluation Président du jury et Andreas Ruediger examinateur interne Professeur à l’INRS-ÉMT Examinateur externe Ricardo Izquierdo Professeur à l’École de technologie supérieure (ETS) Examinateur externe Sasha Omanovic Professeur à l’Université de McGill Directeur de recherche Mohamed Mohamedi Professeur à l’INRS-ÉMT Codirecteur de recherche Shuhui Sun Professeur à l’INRS-ÉMT © Droits réservés de Juan Carlos Abrego Martínez, 4 Juin 2020 ACKNOWLEDGMENTS First and foremost, I would like to thank my supervisor, Prof. Mohamed Mohamedi for offering me the opportunity to undertake my PhD studies in his research group, for providing the tools and trusting me to carry out this project and for the excellent guidance and support throughout this period. I also express my gratitude to my co-supervisor, Prof. Shuhui Sun for closely following the research progress and for his valuable contribution for accomplishing my PhD project. I thank the Jury members, Prof. Andreas Ruediger, Prof. Ricardo Izquierdo and Prof. Sasha Omanovic for agreeing and taking the time to review and evaluate this work. I would like to thank my group colleagues, Youling Wang, Alonso Moreno, Haixia Wang, Naser Mohammadi, Xiaoying Zheng, Khawtar Hasan and Soraya Bouachma, for sharing their knowledge and experience through discussions and experiments in the laboratory. -

Co-Laminar Flow Cells for Electrochemical Energy Conversion
Co-laminar flow cells for electrochemical energy conversion by Marc-Antoni Goulet M.Sc. (Physics), McMaster University, 2008 B.Sc., McGill University, 2006 Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the School of Engineering Science Faculty of Applied Sciences Marc-Antoni Goulet 2016 SIMON FRASER UNIVERSITY Summer 2016 Approval Name: Marc-Antoni Goulet Degree: Doctor of Philosophy Title: Co-laminar flow cells for electrochemical energy conversion Examining Committee: Chair: Amr Marzouk Lecturer Erik Kjeang Senior Supervisor Associate Professor Michael Eikerling Supervisor Professor Gary Wang Supervisor Professor Edward Park Internal Examiner Professor Matthew Mench External Examiner Professor Department of Mechanical, Aerospace and Biomedical Engineering University of Tennessee, Knoxville Date Defended/Approved: May 2nd, 2016 ii Abstract A recently developed class of electrochemical cell based on co-laminar flow of reactants through porous electrodes is investigated. New architectures are designed and assessed for fuel recirculation and rechargeable battery operation. Extensive characterization of cells is performed to determine most sources of voltage loss during operation. To this end, a specialized flow cell technique is developed to mitigate mass transport limitations and measure kinetic rates of reaction on flow-through porous electrodes. This technique is used in in conjunction with cyclic voltammetry and electrochemical impedance spectroscopy to evaluate different treatments for enhancing the rates of vanadium redox reactions on carbon paper electrodes. It is determined that surface area enhancements are the most effective way for increasing redox reaction rates and thus a novel in situ flowing deposition method is conceived to achieve this objective at minimal cost. -
DOE Hydrogen Program 2010 Annual Merit Review and Peer Evaluation
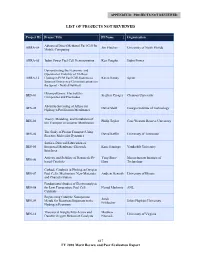
APPENDIX D: PROJECTS NOT REVIEWED LIST OF PROJECTS NOT REVIEWED Project ID Project Title PI Name Organization Advanced Direct Methanol Fuel Cell for ARRA-04 Jim Fletcher University of North Florida Mobile Computing ARRA-05 Jadoo Power Fuel Cell Demonstration Ken Vaughn Jadoo Power Demonstrating the Economic and Operational Viability of 72-Hour ARRA-12 Hydrogen PEM Fuel Cell Systems to Kevin Kenny Sprint Support Emergency Communications on the Sprint - Nextel Network Fluoropolymers, Electrolytes, BES-01 Stephen Creager Clemson University Composites and Electrodes Ab-initio Screening of Alloys for BES-02 David Sholl Georgia Institute of Technology Hydrogen Purification Membranes Theory, Modeling, and Simulation of BES-03 Philip Taylor Case Western Reserve University Ion Transport in Ionomer Membranes The Study of Proton Transport Using BES-04 David Keffer University of Tennessee Reactive Molecular Dynamics Surface-Directed Fabrication of BES-05 Integrated Membrane-Electrode Kane Jennings Vanderbilt University Interfaces Activity and Stability of Nanoscale Pt- Yang Shao- Massachusetts Institute of BES-06 based Catalysts Horn Technology Cathode Catalysis in Hydrogen/Oxygen BES-07 Fuel Cells: Mechanism, New Materials, Andrew Gewirth University of Illinois and Characterization Fundamental Studies of Electrocatalysis BES-08 for Low Temperature Fuel Cell Nenad Markovic ANL Catalysts Engineering Catalytic Nanoporous Jonah BES-09 Metals for Reactions Important to the Johns Hopkins University Erlebacher Hydrogen Economy Theoretical Insights Into -

Membraneless Hydrogen Bromine Laminar Flow Battery for Large
Membraneless Hydrogen Bromine Laminar Flow Battery for Large-Scale Energy Storage by William Allan Braff Submitted to the Department of Mechanical Engineering in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY February 2014 c Massachusetts Institute of Technology 2014. All rights reserved. Author.............................................................. Department of Mechanical Engineering December 19, 2013 Certified by. Cullen R. Buie Assistant Professor of Mechanical Engineering Thesis Supervisor Certified by. Martin Z. Bazant Professor of Chemical Engineering and Mathematics Thesis Supervisor Accepted by . David E. Hardt Chairman, Department Committee on Graduate Theses 2 Membraneless Hydrogen Bromine Laminar Flow Battery for Large-Scale Energy Storage by William Allan Braff Submitted to the Department of Mechanical Engineering on December 19, 2013, in partial fulfillment of the requirements for the degree of Doctor of Philosophy Abstract Electrochemical energy storage systems have been considered for a range of potential large-scale energy storage applications. These applications vary widely, both in the order of magnitude of energy storage that is required and the rate at which energy must be charged and discharged. One such application aids the integration of renew- able energy technologies onto the electrical grid by shifting the output from renewable energy resources to periods of high demand, relaxing transmission and distribution requirements and reducing the need for fossil fuel burning plants. Although the mar- ket need for such solutions is well known, existing technologies are still too expensive to compete with conventional combustion-based solutions. In this thesis, the hydrogen bromine laminar flow battery (HBFLB) is proposed and examined for its potential to provide low cost energy storage using the rapid reaction kinetics of hydrogen-bromine reaction pairs and a membrane-less laminar flow battery architecture. -

Fabrication of Microfluidic Devices with Application to Membraneless Fuel Cells by Jon Mckechnie B.Eng. Mcmaster University
Fabrication of Microfluidic Devices with Application to Membraneless Fuel Cells by Jon McKechnie B.Eng. McMaster University, 2004 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF APPLIED SCIENCE in the Department of Mechanical Engineering © Jon McKechnie, 2006 University of Victoria All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. ii Fabrication of Microfluidic Devices with Application to Membraneless Fuel Cells by Jon McKechnie B.Eng. McMaster University, 2004 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF APPLIED SCIENCE in the Department of Mechanical Engineering Supervisory Committee: Dr. David Sinton (Mechanical Engineering)____________________________________ Supervisor Dr. Ned Djilali (Mechanical Engineering)______________________________________ Departmental Member Dr. David Levin (Biology)__________________________________________________ Outside Member iii Supervisory Committee: Dr. David Sinton (Mechanical Engineering)____________________________________ Supervisor Dr. Ned Djilali (Mechanical Engineering)______________________________________ Departmental Member Dr. David Levin (Biology)__________________________________________________ Outside Member ABSTRACT This thesis is part of an ongoing collaborative research project focused on the development of microstructured enzymatic fuel cells. Both enzymatic fuel cells and co- laminar fuel cells are, more -

A Review on Membraneless Laminar Flow-Based Fuel Cells
international journal of hydrogen energy xxx (2011) 1e20 Available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/he Review A review on membraneless laminar flow-based fuel cells Seyed Ali Mousavi Shaegh, Nam-Trung Nguyen*, Siew Hwa Chan School of Mechanical and Aerospace Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore article info abstract Article history: The review article provides a methodical approach for understanding membraneless Received 19 October 2010 laminar flow-based fuel cells (LFFCs), also known as microfluidic fuel cells. Membraneless Received in revised form LFFCs benefit from the lamination of multiple streams in a microchannel. The lack of 9 January 2011 convective mixing leads to a well-defined liquideliquid interface. Usually, anode and Accepted 12 January 2011 cathode are positioned at both sides of the interface. The liquideliquid interface is Available online xxx considered as a virtual membrane and ions can travel across the channel to reach the other side and complete the ionic conduction. The advantage of membraneless LFFC is the lack Keywords: of a physical membrane and the related issues of membrane conditioning can be elimi- Microfluidic nated or becomes less important. Based on the electrode architectures, membraneless Fuel cell LFFCs in the literature can be categorized into three main types: flow-over design with Laminar flow planar electrodes, flow-through design with three-dimensional porous electrodes, and Membraneless membraneless LFFCs with air-breathing cathode. Since this paper focuses on reviewing the Review design considerations of membraneless LFFCs, a concept map is provided for under- standing the cross-related problems. The impacts of flow and electrode architecture on cell performance and fuel utilization are discussed. -

Pressure Retarded Osmosis 1St Edition Free Download
FREE PRESSURE RETARDED OSMOSIS 1ST EDITION PDF Eng Khaled Touati | 9780128123157 | | | | | [PDF] Pressure Retarded Osmosis eBook Download Full HQ Get free access to the library by create an account, fast download and ads free. Summary : Pressure Retarded Osmosis: Renewable Energy Generation and Pressure Retarded Osmosis 1st edition offers the first comprehensive resource on this method of generating renewable energy. Khaled Touati and the team of editors combine their expertise with contributions from other leaders in the field to create this well-rounded resource, which discusses and analyses this novel method of creating a controllable renewable energy. The promises of the PRO technique are first clearly presented and explained, and the authors then provide a comprehensive analysis of the issues that remain such as Concentration Polarization, Membrane Deformation, and Pressure Retarded Osmosis 1st edition Salt Diffusion. Possible solutions to these issues which often restrict industrial implementation are then discussed to mitigate these detrimental effects, and there is also an emphasis on the recovery of energy from desalination processes using PRO, which is able to reduce energy Pressure Retarded Osmosis 1st edition and make it more economically and environmentally efficient. Combines research with experience Pressure Retarded Osmosis 1st edition deliver a complete resource on Pressure Retarded Osmosis Discusses all areas of PRO in detail Offers solutions to problems commonly experienced and summarizes each method with a clear and concise conclusion Includes case studies from the Great Salt Lake U. Summary : Osmotic energy can be effectively Pressure Retarded Osmosis 1st edition through pressure retarded osmosis PRO which is the most widely investigated technology due to its greater efficiency and higher power density output and effective membranes are the heart of the PRO technology. -

1 a Review on Unitized Regenerative Fuel Cell Technologies, Part B
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Heriot Watt Pure A review on unitized regenerative fuel cell technologies, part B: unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell Yifei Wanga, Dennis Y.C. Leunga, Jin Xuana,b, Huizhi Wanga,b aDepartment of Mechanical Engineering, The University of Hong Kong, Pokfulam Road, Hong Kong bSchool of Engineering & Physical Sciences, Heriot-Watt University, Edinburgh EH14 4AS, United Kingdom Corresponding author: Dennis Y.C. Leung Tel: (852) 28597911 Fax: (852) 28585415 Email: [email protected] Abstract In part A of this review, we have introduced the research progress and application status of unitized regenerative proton exchange membrane fuel cells. In addition to this proton exchange membrane (PEM)-based unitized regenerative fuel cell (URFC), other URFC technologies with different electrolytes have also been reported in the literature, which are the emphasis of this part of review. Unitized regenerative alkaline fuel cells (UR-AFC) have long been utilized for aerospace applications, while the recent development of anion exchange membrane (AEM) has stimulated their further development, especially on the AEM-based UR-AFCs. Vast research works have been reported on the bifunctional oxygen catalyst development, while the latest UR-AFC prototypes are also briefly introduced. Despite of their potential cost-efficiency and better 1 reactivity, cell performance and round-trip efficiency of the current UR-AFCs are still lower than their PEM-based counterparts. Unitized regenerative solid oxide fuel cell, or more commonly cited as reversible solid oxide fuel cell (RSOFC), is a high-temperature URFC technology with superior performance and reversibility. -
Microfluidic Platforms for the Investigation of Fuel Cell Catalysts and Electrodes
MICROFLUIDIC PLATFORMS FOR THE INVESTIGATION OF FUEL CELL CATALYSTS AND ELECTRODES BY FIKILE R. BRUSHETT DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemical Engineering in the Graduate College of the University of Illinois at Urbana-Champaign, 2010 Urbana, Illinois Doctoral Committee: Professor Paul J.A. Kenis, Chair Professor Rich I. Masel Professor Andrzej Wieckowski Professor Edmund G. Seebauer Abstract A clear need exists for novel approaches to producing and utilizing energy in more efficient ways, in light of society’s ever increasing demand as well as growing concerns with respect to climate change related to CO2 emissions. The development of low temperature fuel cell technologies will continue to play an important role in many alternative energy conversion strategies, especially for portable electronics and automotive applications. However, widespread commercialization of fuel cell technologies has yet to be achieved due to a combination of high costs, poor durability and, system performance limitations (Chapter 1). Developing a better understanding of the complex interplay of electrochemical, transport, and degradation processes that govern the performance and durability of novel fuel cell components, particularly catalysts and electrodes, within operating fuel cells is critical to designing robust, inexpensive configurations that are required for commercial introduction. Such detailed in-situ investigations of individual electrode processes are complicated by other factors such as water management, uneven performance across electrodes, and temperature gradients. Indeed, too many processes are interdependent on the same few variable parameters, necessitating the development of novel analytical platforms with more degrees of freedom. Previously, membraneless microfluidic fuel cells have been developed to address some of the aforementioned fuel cell challenges (Chapter 2). -

Microfluidic Fuel Cell for Off‑The‑Grid Applications
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Microfluidic fuel cell for off‑the‑grid applications Seyed Ali Mousavi Shaegh 2012 Seyed, A. M. S. (2012). Microfluidic fuel cell for off‑the‑grid applications. Doctoral thesis, Nanyang Technological University, Singapore. https://hdl.handle.net/10356/50777 https://doi.org/10.32657/10356/50777 Downloaded on 25 Sep 2021 01:14:44 SGT Microfluidic Fuel Cell for off-the-grid Applications Seyed Ali Mousavi Shaegh School of Mechanical and Aerospace Engineering A thesis submitted to Nanyang Technological University in partial fulfillment of the requirements for the degree of Doctor of Philosophy 2012 Abstract The present doctoral thesis studies air-breathing microfluidic fuel cells with separated fuel and electrolyte streams as well as a membraneless fuel cell with selective electrodes. In order to gain more insight into the physio-chemical reactions, numerical simulation of the in-house developed air-breathing microfluidic fuel cell is formulated and solved using COMSOL Multiphysics. The results from the simulation show that fuel stream at the anode side and its interaction with the electrolyte stream has significant impact on the total fuel cell performance. As the first step for improving the hydrodynamic manipulation of the fuel stream, a flow-through porous anode is introduced. The effects of flow architecture on fuel utilization and the whole cell performance are investigated. Experimental results show that the flow-through porous anode improves the cell current in a long-term performance test as compared to the conventional design with flow-over planar anode. -

Electrolysis Without Membranes
Electrolysis without Membranes Glen O’Neil, Oyin Talabi, David Brown, Cory Christian, Ji Qi, Jack Davis, Anna Dorfi Dan Esposito Department of Chemical Engineering Lenfest Center for Sustainable Energy Columbia University Closing the Carbon Cycle Conference Tempe, AZ, September 29th, 2016 9/29/16 D. Esposito, Closing the Carbon Cycle 1 Mission The mission of the Lenfest Center for Sustainable Energy (LCSE) is to advance science and develop innovative technologies that provide sustainable energy for all humanity while maintaining the stability of the Earth’s natural systems. Research Themes Six interconnected, topical research areas that fall under the overall theme of sustainable energy conversion and utilization pathways: I. Novel Materials/Nanotechnology for energy conversion, utilization and storage with a reduced environmental footprint. II. Novel Reaction Pathways for sustainable energy materials conversion throughout the engineered and natural elemental cycles, including innovative device development (e.g. 3-D printed reactor systems). III. Catalysis for novel reaction pathways. IV. Separations using smart, multi-functional materials for sustainable energy and materials. V. Energy Storage and Systems Integration for optimized deployment of renewable energy. VI. Earth Systems for sustainable energy extraction, conversion and waste storage (e.g. waterless or CO2-rich fracking of shale, enhanced oil recovery, geothermal heat recovery with integrated CO2 conversion, and CO2 storage). 9/29/16 D. Esposito, Closing the Carbon Cycle 2 Electrochemical Production of Fuels (+) (-) H2O Electrolysis V - Red.: + - e 2H +2e → H2 - Ox.: - + e H2O →2e 2H +0.5O2 Overall: H2O → H2 +0.5O2 O2 H+ H2 H2O Anode Cathode membrane Side-view of PEM electrolysis cell. 9/29/16 D. -

Erik Kjeang Microfl Uidic Fuel Cells and Batteries
SPRINGER BRIEFS IN ENERGY Erik Kjeang Microfl uidic Fuel Cells and Batteries 123 SpringerBriefs in Energy For further volumes: http://www.springer.com/series/8903 Erik Kjeang Microfl uidic Fuel Cells and Batteries Erik Kjeang Fuel Cell Research Laboratory School of Mechatronic Systems Engineering Simon Fraser University Surrey , BC , Canada ISSN 2191-5520 ISSN 2191-5539 (electronic) ISBN 978-3-319-06345-4 ISBN 978-3-319-06346-1 (eBook) DOI 10.1007/978-3-319-06346-1 Springer Cham Heidelberg New York Dordrecht London Library of Congress Control Number: 2014939516 © The Author(s) - SpringerBriefs 2014 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifi cally for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. Permissions for use may be obtained through RightsLink at the Copyright Clearance Center. Violations are liable to prosecution under the respective Copyright Law.