Crustacean Proteases and Their Application in Debridement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

A Chymotrypsin from the Digestive Tract of California Spiny Lobster, Panulirus Interruptus: Purification and Biochemical Characterization
A chymotrypsin from the Digestive Tract of California Spiny Lobster, Panulirus interruptus: Purification and Biochemical Characterization Betsaida Bibo-Verdugo, Liliana Rojo- Arreola, Maria A. Navarrete-del-Toro & Fernando García-Carreño Marine Biotechnology An International Journal Focusing on Marine Genomics, Molecular Biology and Biotechnology ISSN 1436-2228 Volume 17 Number 4 Mar Biotechnol (2015) 17:416-427 DOI 10.1007/s10126-015-9626-z 1 23 Your article is protected by copyright and all rights are held exclusively by Springer Science +Business Media New York. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Mar Biotechnol (2015) 17:416–427 DOI 10.1007/s10126-015-9626-z ORIGINAL ARTICLE A chymotrypsin from the Digestive Tract of California Spiny Lobster, Panulirus interruptus: Purification and Biochemical Characterization Betsaida Bibo-Verdugo1 & Liliana Rojo-Arreola1 & Maria A. Navarrete-del-Toro1 & Fernando García-Carreño1 Received: 13 August 2014 /Accepted: 31 January 2015 /Published online: 16 April 2015 # Springer Science+Business Media New York 2015 Abstract A chymotrypsin was purified from the gastric juice Introduction of California spiny lobster (Panulirus interrutpus), using pre- parative electrophoresis and affinity chromatography on aga- Proteolytic enzymes from the digestive system of crustacean rose-p-aminobenzamidine. -

A-Ailable At
International Journal of Current Advanced Research ISSN: O: 2319-6475, ISSN: P: 2319 – 6505, Impact Factor: SJIF: 5.438 Available Online at www.journalijcar.org Volume 6; Issue 2; February 2017; Page No. 2131-2138 Research Article A COMPARATIVE ANALYSIS OF THERMOPHILIC AND PSYCHROPHILIC METALLOPEPTIDASES: INVOLVEMENT OF BIOINFORMATIC APPROACH Anuprabha1., AbhigyanNath2 and Radha Chaube3* 1,2 3Bioinformatic Division, MahilaMahavidyalya, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India ARTICLEDepartment INFO of Zoology, Institute of Science,ABSTRACT Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India The thermolysin family of enzymes is classified as the M4 family of metallopeptidases. M4 Article History: family comprises numerous zinc-dependent metallopeptidases that hydrolyze peptide Received 9th November, 2016 bonds. Zinc containing peptidases are widely distributed in nature and have important roles Received in revised form 12thDecember, 2016 in many physiological processes. M4 family comprises numerous zinc-dependent Accepted 5th January, 2017 metallopeptidases that hydrolyse peptide bonds. Metallopeptidases were studied for the Published online 28th February, 2017 reason of their great relevance to biology, medicine and biotechnology. In the present study, detailed comparative analyses of both thermophilic and psychrophilic metallopeptidases were performed. The comparative analysis of both thermophilic and Key words: psychrophilic metallopeptidases was performed by taking sequence parameters such as Thermophilic, Psychrophilic, amino acid composition, amino acid property group composition, physico-chemical Metallopeptidases, amino acid residues, properties, secondary structure content. From comparison between the two groups, it was analysis found that Gln, Asn, Ser, Thr, and His are significantly lower in thermophilic metallopeptidase. Positively charged residues (Lys, Arg and Glu) are more significant in thermophilic metallopeptidases than in psychrophilic metallopeptidases. -
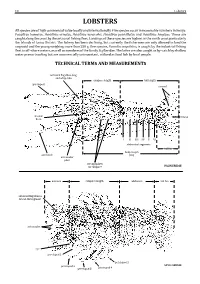
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

(12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002
USOO6395889B1 (12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002 (54) NUCLEIC ACID MOLECULES ENCODING WO WO-98/56804 A1 * 12/1998 ........... CO7H/21/02 HUMAN PROTEASE HOMOLOGS WO WO-99/0785.0 A1 * 2/1999 ... C12N/15/12 WO WO-99/37660 A1 * 7/1999 ........... CO7H/21/04 (75) Inventor: fish E. Robison, Wilmington, MA OTHER PUBLICATIONS Vazquez, F., et al., 1999, “METH-1, a human ortholog of (73) Assignee: Millennium Pharmaceuticals, Inc., ADAMTS-1, and METH-2 are members of a new family of Cambridge, MA (US) proteins with angio-inhibitory activity', The Journal of c: - 0 Biological Chemistry, vol. 274, No. 33, pp. 23349–23357.* (*) Notice: Subject to any disclaimer, the term of this Descriptors of Protease Classes in Prosite and Pfam Data patent is extended or adjusted under 35 bases. U.S.C. 154(b) by 0 days. * cited by examiner (21) Appl. No.: 09/392, 184 Primary Examiner Ponnathapu Achutamurthy (22) Filed: Sep. 9, 1999 ASSistant Examiner William W. Moore (51) Int. Cl." C12N 15/57; C12N 15/12; (74) Attorney, Agent, or Firm-Alston & Bird LLP C12N 9/64; C12N 15/79 (57) ABSTRACT (52) U.S. Cl. .................... 536/23.2; 536/23.5; 435/69.1; 435/252.3; 435/320.1 The invention relates to polynucleotides encoding newly (58) Field of Search ............................... 536,232,235. identified protease homologs. The invention also relates to 435/6, 226, 69.1, 252.3 the proteases. The invention further relates to methods using s s s/ - - -us the protease polypeptides and polynucleotides as a target for (56) References Cited diagnosis and treatment in protease-mediated disorders. -

The Extracellular Proteases Produced by Vibrio Parahaemolyticus
World Journal of Microbiology and Biotechnology (2018) 34:68 https://doi.org/10.1007/s11274-018-2453-4 REVIEW The extracellular proteases produced by Vibrio parahaemolyticus George Osei‑Adjei1 · Xinxiang Huang1 · Yiquan Zhang1 Received: 20 February 2018 / Accepted: 8 May 2018 © Springer Science+Business Media B.V., part of Springer Nature 2018 Abstract Vibrio parahaemolyticus, a Gram-negative bacterium, inhabits marine and estuarine environments and it is a major pathogen responsible globally for most cases of seafood-associated gastroenteritis in humans and acute hepatopancreatic necrosis syn- drome in shrimps. There has been a dramatic worldwide increase in V. parahaemolyticus infections over the last two decades. The pathogenicity of V. parahaemolyticus has been linked to the expression of different kinds of virulence factors including extracellular proteases, such as metalloproteases and serine proteases. V. parahaemolyticus expresses the metalloproteases; PrtV, VppC, VPM and the serine proteases; VPP1/Protease A, VpSP37, PrtA. Extracellular proteases have been identified as potential virulence factors which directly digest many kinds of host proteins or indirectly are involved in the processing of other toxic protein factors. This review summarizes findings on the metalloproteases and serine proteases produced by V. parahaemolyticus and their roles in infections. Identifying the role of V. parahaemolyticus virulence-associated extracellular proteases deepens our understanding of diseases caused by this bacterium. Keywords Extracellular protease · Metalloprotease · Serine protease · Vibrio parahaemolyticus · Virulence Introduction incubation period of V. parahaemolyticus is usually 12–24 h post-infection with infection usually occurring in the hot The Gram-negative bacterium, Vibrio parahaemolyticus, summer months because the bacteria is found free swim- lives in marine and estuarine environments and it is a major ming in coastal water or attached to fish and shellfish. -

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

Nexobrid 2 G Powder and Gel for Gel
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. 1. NAME OF THE MEDICINAL PRODUCT NexoBrid 2 g powder and gel for gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 2 g of concentrate of proteolytic enzymes enriched in bromelain, corresponding to 0.09 g/g concentrate of proteolytic enzymes enriched in bromelain after mixing (or 2 g/22 g gel). The proteolytic enzymes are a mixture of enzymes from the stem of Ananas comosus (pineapple plant). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder and gel for gel. The powder is off-white to light tan. The gel is clear and colourless. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications NexoBrid is indicated for removal of eschar in adults with deep partial- and full-thickness thermal burns. 4.2 Posology and method of administration NexoBrid should only be applied by trained healthcare professionals in specialist burn centres. Posology 2 g NexoBrid powder in 20 g gel is applied to a burn wound area of 100 cm2. NexoBrid should not be applied to more than 15% Total Body Surface Area (TBSA) (see also section 4.4, Coagulopathy). NexoBrid should be left in contact with the burn for a duration of 4 hours. There is very limited information on the use of NexoBrid on areas where eschar remained after the first application. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110747 A1 Ramseier Et Al
US 200601 10747A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110747 A1 Ramseier et al. (43) Pub. Date: May 25, 2006 (54) PROCESS FOR IMPROVED PROTEIN (60) Provisional application No. 60/591489, filed on Jul. EXPRESSION BY STRAIN ENGINEERING 26, 2004. (75) Inventors: Thomas M. Ramseier, Poway, CA Publication Classification (US); Hongfan Jin, San Diego, CA (51) Int. Cl. (US); Charles H. Squires, Poway, CA CI2O I/68 (2006.01) (US) GOIN 33/53 (2006.01) CI2N 15/74 (2006.01) Correspondence Address: (52) U.S. Cl. ................................ 435/6: 435/7.1; 435/471 KING & SPALDING LLP 118O PEACHTREE STREET (57) ABSTRACT ATLANTA, GA 30309 (US) This invention is a process for improving the production levels of recombinant proteins or peptides or improving the (73) Assignee: Dow Global Technologies Inc., Midland, level of active recombinant proteins or peptides expressed in MI (US) host cells. The invention is a process of comparing two genetic profiles of a cell that expresses a recombinant (21) Appl. No.: 11/189,375 protein and modifying the cell to change the expression of a gene product that is upregulated in response to the recom (22) Filed: Jul. 26, 2005 binant protein expression. The process can improve protein production or can improve protein quality, for example, by Related U.S. Application Data increasing solubility of a recombinant protein. Patent Application Publication May 25, 2006 Sheet 1 of 15 US 2006/0110747 A1 Figure 1 09 010909070£020\,0 10°0 Patent Application Publication May 25, 2006 Sheet 2 of 15 US 2006/0110747 A1 Figure 2 Ester sers Custer || || || || || HH-I-H 1 H4 s a cisiers TT closers | | | | | | Ya S T RXFO 1961. -

Development of Granulation Tissue Mimetic Scaffolds for Skin Healing
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 10-4-2018 12:30 PM Development Of Granulation Tissue Mimetic Scaffolds For Skin Healing Adam Hopfgartner The University of Western Ontario Supervisor Hamilton, Douglas W. The University of Western Ontario Co-Supervisor Pickering, J. Geoffrey. The University of Western Ontario Graduate Program in Biomedical Engineering A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Engineering Science © Adam Hopfgartner 2018 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Biomaterials Commons, and the Molecular, Cellular, and Tissue Engineering Commons Recommended Citation Hopfgartner, Adam, "Development Of Granulation Tissue Mimetic Scaffolds For Skin Healing" (2018). Electronic Thesis and Dissertation Repository. 5767. https://ir.lib.uwo.ca/etd/5767 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract Impaired skin healing is a significant and growing clinical concern, particularly in relation to diabetes, venous insufficiency and immobility. Previously, we developed electrospun scaffolds for the delivery of periostin (POSTN) and connective tissue growth factor 2 (CCN2), matricellular proteins involved in the proliferative phase of healing. This study aimed to design and validate a novel electrosprayed coaxial microsphere for the encapsulation of fibroblast growth factor 9 (FGF9), as a component of the POSTN/CCN2 scaffold, to promote angiogenic stability during wound healing. For the first time, we observed a pro-proliferative effect of FGF9 on human dermal fibroblasts (HDF) in vitro, indicating a potential cellular mechanism of action during wound healing.